VDAC1 Inhibition Protects Against Noise-Induced Hearing Loss via the PINK1/Parkin Pathway
- PMID: 40285415
- PMCID: PMC12032401
- DOI: 10.1111/cns.70410
VDAC1 Inhibition Protects Against Noise-Induced Hearing Loss via the PINK1/Parkin Pathway
Abstract
Aims: This study examined the effect of 4,4'-diisothiocyanostilbene-2,2'-disulfonic acid (DIDS), an anion channel blocker of voltage-dependent anion channel 1 (VDAC1), on noise-induced hearing loss (NIHL) and its underlying mechanisms.
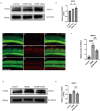
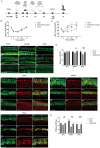
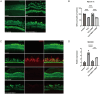
Methods: Cochlear explants and House Ear Institute-Organ of Corti 1 (HEI-OC1) cells were used to assess the effect of DIDS in vitro. Auditory brainstem responses were used to assess auditory functions in mice. Immunofluorescence staining of myosin 7a and CTBP2 were used to examine hair cells and synaptic ribbons. The accumulation of reactive oxygen species (ROS) was measured by 4-HNE staining. The gene expression changes of cochlea were analyzed using RNA sequencing.
Results: DIDS reduced the levels of ROS in cochlear explants and attenuated cell death caused by hydrogen peroxide in both cochlear explants and HEI-OC1 cells. In C57BL/6 mice, DIDS reduced ROS generation and tumor necrosis factor-α induced by noise exposure, thereby protecting outer hair cells and inner hair cell synaptic ribbons from noise-induced damage through a mechanism involving the PINK1/Parkin signaling pathway. The preventive effect of DIDS in cochlear explants was eliminated by mitophagy inhibition.
Conclusion: VDAC1 inhibition enhances mitophagy in cochlear hair cells, playing a critical role in defending against oxidative stress and inflammation. Downregulation of VDAC1 may thus be considered a therapeutic strategy for preventing cochlear hair cell damage and reducing NIHL.
Keywords: 4,4′‐Diisothiocyanostilbene‐2,2′‐disulfonic acid; autophagy; hair cell; noise‐induced hearing loss; reactive oxygen species.
© 2025 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
G.E.O. data set: Gene expression data (GSE137299 profiling data) were downloaded from Gene Expression Omnibus (
Animal studies: All animal experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Shanghai Sixth People's Hospital, affiliated with Shanghai Jiao Tong University School of Medicine.
The authors declare no conflicts of interest.
Figures





Similar articles
-
Calcitriol alleviates noise-induced hearing loss by regulating the ATF3/DUSP1 signalling pathway.Ecotoxicol Environ Saf. 2024 Oct 1;284:116906. doi: 10.1016/j.ecoenv.2024.116906. Epub 2024 Aug 24. Ecotoxicol Environ Saf. 2024. PMID: 39182283
-
Noise-Induced Loss of Hair Cells and Cochlear Synaptopathy Are Mediated by the Activation of AMPK.J Neurosci. 2016 Jul 13;36(28):7497-510. doi: 10.1523/JNEUROSCI.0782-16.2016. J Neurosci. 2016. PMID: 27413159 Free PMC article.
-
Norepinephrine protects against cochlear outer hair cell damage and noise-induced hearing loss via α2A-adrenergic receptor.BMC Neurosci. 2024 Jan 30;25(1):5. doi: 10.1186/s12868-024-00845-4. BMC Neurosci. 2024. PMID: 38291397 Free PMC article.
-
Inner Ear Hair Cell Protection in Mammals against the Noise-Induced Cochlear Damage.Neural Plast. 2018 Jul 15;2018:3170801. doi: 10.1155/2018/3170801. eCollection 2018. Neural Plast. 2018. PMID: 30123244 Free PMC article. Review.
-
Cellular signaling protective against noise-induced hearing loss – A role for novel intrinsic cochlear signaling involving corticotropin-releasing factor?Biochem Pharmacol. 2015 Sep 1;97(1):1-15. doi: 10.1016/j.bcp.2015.06.011. Epub 2015 Jun 11. Biochem Pharmacol. 2015. PMID: 26074267 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

