Characterizing TLR4 agonist EmT4™ as an anti-Mycobacterium tuberculosis vaccine adjuvant
- PMID: 40285479
- PMCID: PMC12032397
- DOI: 10.1093/immhor/vlaf014
Characterizing TLR4 agonist EmT4™ as an anti-Mycobacterium tuberculosis vaccine adjuvant
Abstract
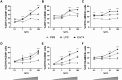
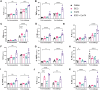
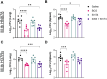
Tuberculosis (TB) is again the deadliest infectious disease globally, and more efficacious vaccines are needed to reduce this mortality. Successful subunit TB vaccines need antigens and adjuvants that are immunogenic, inexpensive, and accessible. Here we evaluated the potential of synthetically produced Monophosphoryl lipid A (SyMLP), a TLR4-agonist, formulated in an oil-in-water emulsion (EmT4™) in combination with selected fusion proteins, to drive an effective vaccine-mediated immunogenic response in C57BL/6 mice against Mycobacterium tuberculosis (M.tb) HN878 and H37Rv challenge. We first observed that EmT4™ enhances activation of C57BL/6 bone-marrow derived macrophages and dendritic cells measured by CD40, CD86, and MHCII expression by flow cytometry. EmT4™ did not induce safety signals in a scaled tolerability study. In immunogenicity studies, mice immunized 3 times 3 weeks apart with ID93 antigen + EmT4™ produced a significantly higher magnitude of circulating proinflammatory cytokines and ID93-specific immunoglobulin G (IgG) antibodies pre- and post-challenge with M.tb than saline control animals. Ex vivo ID93 restimulated splenocytes and lung cells elicited significant polyfunctional CD4+ T-helper 1 responses. Importantly, ID93 + EmT4™ immunizations significantly reduced bacterial burden in C57BL/6 mice 4 weeks post-challenge. Interestingly, EmT4™ paired with a next generation protein fusion ID91 also afforded prophylactic protection against M.tb HN878 challenge in both young (6 to 8 wk) and aged (20 mo) immunocompromised Beige mice. These protection and immunogenicity findings suggest that synthetically derived EmT4™ adjuvant is not only suitable to help backfill the preclinical TB vaccine candidate pipeline but is also suitable for the needs of the global community.
Keywords: Mycobacterium tuberculosis; TB; adjuvant; mouse model; vaccine.
© The Author(s) 2025. Published by Oxford University Press on behalf of The American Association of Immunologists.
Conflict of interest statement
D.C. and S.A.G. work for PAI Life Sciences Inc., where EmT4™ is manufactured. The remaining authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






References
-
- World Health Organization. Global TB report. 2022. Accessed October, 2022. https://www.who.int/teams/global-programme-on-tuberculosis-and-lung-heal...
-
- World Health Organization. Global tuberculosis report. 2023. Accessed November, 2023. https://www.who.int/teams/global-programme-on-tuberculosis-and-lung-heal...
-
- World Health Organization. Global tuberculosis report. 2024. Accessed October, 2024. https://www.who.int/teams/global-programme-on-tuberculosis-and-lung-heal...
-
- Mangtani P et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58:470–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

