LncRNA-MEG3 Regulates Muscle Mass and Metabolic Homeostasis by Facilitating SUZ12 Liquid-Liquid Phase Separation
- PMID: 40285575
- PMCID: PMC12199413
- DOI: 10.1002/advs.202417715
LncRNA-MEG3 Regulates Muscle Mass and Metabolic Homeostasis by Facilitating SUZ12 Liquid-Liquid Phase Separation
Abstract
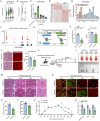
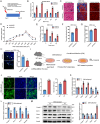
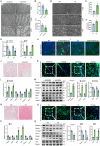
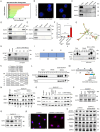
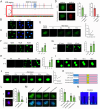
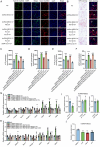
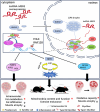
Skeletal muscle plays a crucial role in maintaining motor function and metabolic homeostasis, with its loss or atrophy leading to significant health consequences. Long non-coding RNAs (lncRNAs) have emerged as key regulators in muscle biology; however, their precise roles in muscle function and pathology remain to be fully elucidated. This study demonstrates that lncRNA maternally expressed gene 3 (MEG3) is preferentially expressed in slow-twitch muscle fibers and dynamically regulated during muscle development, aging, and in the context of Duchenne muscular dystrophy (DMD). Using both loss- and gain-of-function mice models, this study shows that lncRNA-MEG3 is critical for preserving muscle mass and function. Its depletion leads to muscle atrophy, mitochondrial dysfunction, and impaired regenerative capacity, while overexpression enhances muscle mass, increases oxidative muscle fiber content, and improves endurance. Notably, lncRNA-MEG3 overexpression in MDX mice significantly alleviates muscle wasting and adipose tissue infiltration. Mechanistically, this study uncovers a novel interaction between lncRNA-MEG3 and the polycomb repressive complex 2 (PRC2), where lncRNA-MEG3 binds to SUZ12 polycomb repressive complex 2 subunit (Suz12), stabilizes PRC2, facilitates SUZ12 liquid-liquid phase separation (LLPS), and regulates the epigenetic modulation of four and a half lim domains 3 (Fhl3) and ring finger protein 128 (Rnf128). These findings not only highlight the crucial role of lncRNA-MEG3 in muscle homeostasis but also provide new insights into lncRNA-based therapeutic strategies for muscle-related diseases.
Keywords: SUZ12 LLPS; fat infiltration; lncRNA‐MEG3; muscle atrophy; oxidative muscle fibers.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Inhibition of mitochondrial fission protein Drp1 ameliorates skeletal myopathy in the D2-mdx model of Duchenne muscular dystrophy.Am J Physiol Cell Physiol. 2025 Jul 1;329(1):C307-C324. doi: 10.1152/ajpcell.01009.2024. Epub 2025 Jun 16. Am J Physiol Cell Physiol. 2025. PMID: 40522885 Free PMC article.
-
Long non-coding RNAs Kcnq1ot1 and Lncpint are involved in skeletal muscle atrophy induced by the space exposome.J Physiol. 2025 Jul;603(14):3973-3986. doi: 10.1113/JP288987. Epub 2025 Jun 29. J Physiol. 2025. PMID: 40581981 Free PMC article.
-
Exploring lncRNA-Mediated Mechanisms in Muscle Regulation and Their Implications for Duchenne Muscular Dystrophy.Int J Mol Sci. 2025 Jun 24;26(13):6032. doi: 10.3390/ijms26136032. Int J Mol Sci. 2025. PMID: 40649811 Free PMC article. Review.
-
Umbelliferone attenuates diabetic sarcopenia by modulating mitochondrial quality and the ubiquitin-proteasome system.Phytomedicine. 2025 Aug;144:156930. doi: 10.1016/j.phymed.2025.156930. Epub 2025 May 31. Phytomedicine. 2025. PMID: 40483791
-
Corticosteroids for the treatment of Duchenne muscular dystrophy.Cochrane Database Syst Rev. 2016 May 5;2016(5):CD003725. doi: 10.1002/14651858.CD003725.pub4. Cochrane Database Syst Rev. 2016. PMID: 27149418 Free PMC article.
References
-
- Fatica A., Bozzoni I., Nat. Rev. Genet. 2014, 15, 7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
