Intelligent Manufacturing for Osteoarthritis Organoids
- PMID: 40285592
- PMCID: PMC12240648
- DOI: 10.1111/cpr.70043
Intelligent Manufacturing for Osteoarthritis Organoids
Abstract
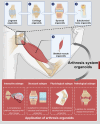
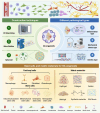
Osteoarthritis (OA) is the most prevalent degenerative joint disease worldwide, imposing a substantial global disease burden. However, its pathogenesis remains incompletely understood, and effective treatment strategies are still lacking. Organoid technology, in which stem cells or progenitor cells self-organise into miniature tissue structures under three-dimensional (3D) culture conditions, provides a promising in vitro platform for simulating the pathological microenvironment of OA. This approach can be employed to investigate disease mechanisms, carry out high-throughput drug screening and facilitate personalised therapies. This review summarises joint structure, OA pathogenesis and pathological manifestations, thereby establishing the disease context for the application of organoid technology. It then examines the components of the arthrosis organoid system, specifically addressing cartilage, subchondral bone, synovium, skeletal muscle and ligament organoids. Furthermore, it details various strategies for constructing OA organoids, including considerations of cell selection, pathological classification and fabrication techniques. Notably, this review introduces the concept of intelligent manufacturing of OA organoids by incorporating emerging engineering technologies such as artificial intelligence (AI) into the organoid fabrication process, thereby forming an innovative software and hardware cluster. Lastly, this review discusses the challenges currently facing intelligent OA organoid manufacturing and highlights future directions for this rapidly evolving field. By offering a comprehensive overview of state-of-the-art methodologies and challenges, this review anticipates that intelligent, automated fabrication of OA organoids will expedite fundamental research, drug discovery and personalised translational applications in the orthopaedic field.
Keywords: arthrosis; artificial intelligence; cartilage; in vitro modelling; intelligent manufacturing; osteoarthritis organoids.
© 2025 The Author(s). Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- GBD 2021 Osteoarthritis Collaborators , “Global, Regional, and National Burden of Osteoarthritis, 1990–2020 and Projections to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021,” Lancet Rheumatology 5, no. 9 (2023): e508–e522, 10.1016/s2665-9913(23)00163-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

