Tumoroid Model Reveals Synergistic Impairment of Metabolism by Iron Chelators and Temozolomide in Chemo-Resistant Patient-derived Glioblastoma Cells
- PMID: 40285641
- PMCID: PMC12120723
- DOI: 10.1002/advs.202412505
Tumoroid Model Reveals Synergistic Impairment of Metabolism by Iron Chelators and Temozolomide in Chemo-Resistant Patient-derived Glioblastoma Cells
Erratum in
-
Correction to "Tumoroid Model Reveals Synergistic Impairment of Metabolism by Iron Chelators and Temozolomide in Chemo-Resistant Patient-derived Glioblastoma Cells".Adv Sci (Weinh). 2025 Dec;12(47):e19458. doi: 10.1002/advs.202519458. Epub 2025 Nov 19. Adv Sci (Weinh). 2025. PMID: 41255256 Free PMC article. No abstract available.
Abstract
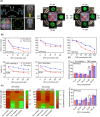
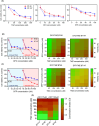
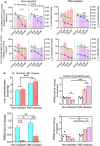
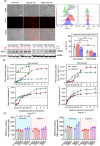
Chemoresistance poses a significant clinical challenge in managing glioblastoma (GBM), limiting the long-term success of traditional treatments. Here, a 3D tumoroid model is used to investigate the metabolic sensitivity of temozolomide (TMZ)-resistant GBM cells to iron chelation by deferoxamine (DFO) and deferiprone (DFP). This work shows that TMZ-resistant GBM cells acquire stem-like characteristics, higher intracellular iron levels, higher expression of aconitase, and elevated reliance on oxidative phosphorylation and proteins associated with iron metabolism. Using a microphysiological model of GBM-on-a-chip consisting of extracellular matrix (ECM)-incorporated tumoroids, this work demonstrates that the combination of iron chelators with TMZ induces a synergistic effect on an in vitro tumoroid model of newly diagnosed and recurrent chemo-resistant patient-derived GBM and reduced their size and invasion. Investigating downstream metabolic variations reveal reduced intracellular iron, increased reactive oxygen species (ROS), upregulated hypoxia-inducible factor-1α, reduced viability, increased autophagy, upregulated ribonucleotide reductase (RRM2), arrested proliferation, and induced cell death in normoxic TMZ-resistant cells. Hypoxic cells, while showing similar results, display reduced responses to iron deficiency, less blebbing, and an induced autophagic flux, suggesting an adaptive mechanism associated with hypoxia. These findings show that co-treatment with iron chelators and TMZ induces a synergistic effect, making this combination a promising GBM therapy.
Keywords: chemoresistance; glioblastoma; iron metabolism; metabolic sensitivity; tumoroid model.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
Authors declare that they have no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
