First-line pazopanib in patients with metastatic epithelioid hemangioendothelioma: a retrospective single-center analysis
- PMID: 40285941
- PMCID: PMC12033179
- DOI: 10.1007/s00432-025-06208-8
First-line pazopanib in patients with metastatic epithelioid hemangioendothelioma: a retrospective single-center analysis
Abstract
Purpose: Epithelioid hemangioendothelioma (EHE) represents an ultra-rare, translocated vascular sarcoma with a heterogeneous course of disease. The optimal systemic treatment for patients with advanced EHE remains unclear. We sought to evaluate the value of pazopanib (PAZ) as a first-line treatment in metastatic EHE.
Methods: Thirteen patients with metastatic EHE and PAZ as a first-line treatment at our institution between 2012 und 2023 were reviewed and analyzed with regard to clinical outcomes.
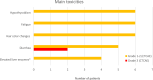
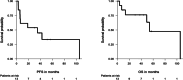
Results: At a median follow-up of 51.4 months, the median progression-free survival (PFS) and overall survival (OS) were 35.1 and 53.8 months, respectively. In patients with documented prior tumor progression (n = 10), the median PFS and OS were 12.6 and 105 months, respectively. In patients with serosal effusion/ systemic symptoms (n = 4), the median PFS and OS were 6.1 and 10.3 months. The clinical benefit rate of the overall cohort was 62% with no complete or partial responses. Two of four patients experienced a reduction of symptoms (pain and ascites reduction/hemoptysis, respectively) under treatment with PAZ. Toxicity was mainly gastrointestinal and manageable with dose reductions. Permanent treatment interruption due to toxicity was necessary in one patient.
Conclusion: This is the first study to systematically report survival outcomes for PAZ as a first-line treatment in patients with metastatic EHE. PAZ is active and safe in patients with metastatic EHE and may be considered as an alternative to sirolimus for specific patient subgroups. RECIST criteria should be questioned for evaluation of treatment response in EHE.
Keywords: Epithelioid hemangioendothelioma; Metastasis; Pazopanib; Rare cancers; Targeted therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The Internal Review Board and the Ethical Review Committee at the Ludwig Maximilians University (LMU) Hospital, Munich, Germany, approved the protocol of this research project (Protocol Nr. 23–0998). The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki. Consent to participate: Not applicable due to the irreversibly anonymized dataset. Competing interests: The authors declare no competing interests.
Figures


References
-
- Agulnik M, Yarber JL, Okuno SH et al (2013) An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol 24:257–263. 10.1093/annonc/mds237 - PubMed
-
- Blay JY, Piperno-Neumann S, Watson S et al (2023) Epithelioid hemangio-endothelioma (EHE) in NETSARC: the nationwide series of 267 patients over 12 years. Eur J Cancer 192. 10.1016/J.EJCA.2023.113262 - PubMed
-
- Cancer Institute N (2017) Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

