Dipalmitoylphosphatidylcholine Lipid Vesicles for Delivering HMB, NMN, and L-Leucine in Sarcopenia Therapy
- PMID: 40286039
- PMCID: PMC11990474
- DOI: 10.3390/molecules30071437
Dipalmitoylphosphatidylcholine Lipid Vesicles for Delivering HMB, NMN, and L-Leucine in Sarcopenia Therapy
Abstract
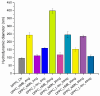
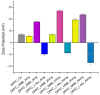
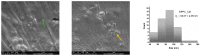
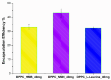
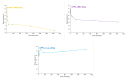
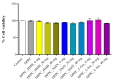
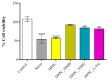
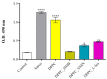
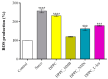
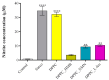
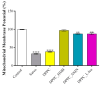
Sarcopenia, characterized by the degeneration of skeletal muscle tissue, has emerged as a significant concern in recent years. This increased awareness stems from advances in research focusing on elderly patients, which have revealed correlations between aging mechanisms and muscle degeneration, beyond the mere fact that tissues age and deteriorate over time. Consequently, the present study aims to address sarcopenia by developing and evaluating DPPC lipid vesicles that encapsulate three distinct drugs: HMB, NMN, and L-Leucine. These drugs are specifically selected for their properties, which facilitate effective interaction with the affected muscle tissue, thereby promoting desired therapeutic effects. Preliminary physicochemical analyses indicate the successful formation of spherical lipid vesicles, characterized by nanometric dimensions and stable membrane integrity. The biological investigations aimed to highlight the potential of DPPC lipid vesicles encapsulating HMB, NMN, and L-Leucine to alleviate sarcopenia-induced cytotoxicity and oxidative stress. Through a comparative evaluation of the three drug formulations, we demonstrate that drug-loaded DPPC vesicles effectively mitigate oxidative damage, preserve mitochondrial function, and maintain cytoskeletal integrity in H2O2-induced C2C12 myotubes, with HMB-loaded vesicles showing the strongest protective effects against muscle degeneration. These findings underscore the therapeutic potential of DPPC-based controlled release systems for sarcopenia treatment and highlight the need for further investigations into their mechanistic role in muscle preservation.
Keywords: DPPC; HMB; L-Leucine; NMN; increased NAD+ levels; lipid vesicles; sarcopenia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Effects of the Combination of β-Hydroxy-β-Methyl Butyrate and R(+) Lipoic Acid in a Cellular Model of Sarcopenia.Molecules. 2020 Apr 30;25(9):2117. doi: 10.3390/molecules25092117. Molecules. 2020. PMID: 32366049 Free PMC article.
-
Leucine and HMB differentially modulate proteasome system in skeletal muscle under different sarcopenic conditions.PLoS One. 2013 Oct 4;8(10):e76752. doi: 10.1371/journal.pone.0076752. eCollection 2013. PLoS One. 2013. PMID: 24124592 Free PMC article.
-
The Potential of β-Hydroxy-β-Methylbutyrate as a New Strategy for the Management of Sarcopenia and Sarcopenic Obesity.Drugs Aging. 2017 Nov;34(11):833-840. doi: 10.1007/s40266-017-0496-0. Drugs Aging. 2017. PMID: 29086232 Review.
-
Beta-Hydroxy-Beta-Methyl Butyrate (HMB): From Experimental Data to Clinical Evidence in Sarcopenia.Curr Protein Pept Sci. 2018;19(7):668-672. doi: 10.2174/1389203718666170529105026. Curr Protein Pept Sci. 2018. PMID: 28554316 Review.
-
Comparison of the anticatabolic effects of leucine and Ca-β-hydroxy-β-methylbutyrate in experimental models of cancer cachexia.Nutrition. 2014 Jul-Aug;30(7-8):807-13. doi: 10.1016/j.nut.2013.11.012. Epub 2013 Dec 4. Nutrition. 2014. PMID: 24984997
Cited by
-
Liposomal and Lipid-Based Drug Delivery Systems: Bridging Gut Microbiota and Pediatric Disorder Treatments.Pharmaceutics. 2025 May 28;17(6):707. doi: 10.3390/pharmaceutics17060707. Pharmaceutics. 2025. PMID: 40574020 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

