Detection of Selected Heavy Metal Ions Using Organic Chromofluorescent Chemosensors
- PMID: 40286044
- PMCID: PMC11990538
- DOI: 10.3390/molecules30071450
Detection of Selected Heavy Metal Ions Using Organic Chromofluorescent Chemosensors
Abstract
Heavy and transition metal (HTM) ions have significant harmful effects on the physical environment and play crucial roles in biological systems; hence, it is crucial to accurately identify and quantify any trace pollution. Molecular sensors which are based on organic molecules employed as optical probes play a crucial role in sensing and detecting toxic metal ions in water, food, air, and biological environments. When appropriate combinations of conduction and selective recognition are combined, fluorescent and colorimetric chemosensors are appealing instruments that enable the selective, sensitive, affordable, portable, and real-time investigation of the possible presence of heavy and transition metal ions. This feature article aims to provide readers with a more thorough understanding of the different methods of synthesis and how they work. As noted in the literature, we will highlight colorimetric and fluorometric sensors based on their receptors into multiple categories for heavy metal ion detection, such as Hg2+, Ag2+, Cd2+, Pb2+, and In3+, and simultaneous multiple-ion detection.
Keywords: biological detection; chemosensor design; colorimetric chemosensors; environmental monitoring; fluorescent chemosensors; heavy and transition metal (HTM) ions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




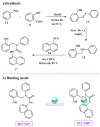

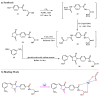





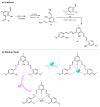



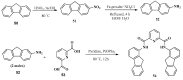



References
-
- Valeur B., Leray I. Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 2000;205:3–40.
-
- Kim H.N., Ren W.X., Kim J.S., Yoon J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012;41:3210–3244. - PubMed
-
- Jonaghani M.Z., Zali-Boeini H. Highly selective fluorescent and colorimetric chemosensor for detection of Hg2+ ion in aqueous media. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017;178:66–70. - PubMed
-
- Xu J.-F., Chen H.-H., Chen Y.-Z., Li Z.-J., Wu L.-Z., Tung C.-H., Yang Q.-Z. A colorimetric and fluorometric dual-modal chemosensor for cyanide in water. Sens. Actuators B Chem. 2012;168:14–19.
-
- Upadhyay S., Singh A., Sinha R., Omer S., Negi K. Colorimetric chemosensors for d-metal ions: A review in the past, present and future prospect. J. Mol. Struct. 2019;1193:89–102.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

