Ti3C2Tx MXene-Based Hybrid Photocatalysts in Organic Dye Degradation: A Review
- PMID: 40286046
- PMCID: PMC11990510
- DOI: 10.3390/molecules30071463
Ti3C2Tx MXene-Based Hybrid Photocatalysts in Organic Dye Degradation: A Review
Abstract
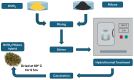
This review provides an overview of the fabrication methods for Ti3C2Tx MXene-based hybrid photocatalysts and evaluates their role in degrading organic dye pollutants. Ti3C2Tx MXene has emerged as a promising material for hybrid photocatalysts due to its high metallic conductivity, excellent hydrophilicity, strong molecular adsorption, and efficient charge transfer. These properties facilitate faster charge separation and minimize electron-hole recombination, leading to exceptional photodegradation performance, long-term stability, and significant attention in dye degradation applications. Ti3C2Tx MXene-based hybrid photocatalysts significantly improve dye degradation efficiency, as evidenced by higher percentage degradation and reduced degradation time compared to conventional semiconducting materials. This review also highlights computational techniques employed to assess and enhance the performance of Ti3C2Tx MXene-based hybrid photocatalysts for dye degradation. It identifies the challenges associated with Ti3C2Tx MXene-based hybrid photocatalyst research and proposes potential solutions, outlining future research directions to address these obstacles effectively.
Keywords: Ti3C2Tx MXene; charge separation; dye degradation; photocatalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Ahmed J., Thakur A., Goyal A. Industrial Wastewater and Its Toxic Effects. In: Shah M.P., editor. Biological Treatment of Industrial Wastewater. The Royal Society of Chemistry; London, UK: 2021. pp. 1–14.
-
- López-Ahumada E., Salazar-Hernández M., Talavera-López A., Solis-Marcial O.J., Hernández-Soto R., Ruelas-Leyva J.P., Hernández J.A. Removal of Anionic and Cationic Dyes Present in Solution Using Biomass of Eichhornia Crassipes as Bioadsorbent. Molecules. 2022;27:6442. doi: 10.3390/molecules27196442. - DOI - PMC - PubMed
-
- Salleh M.A.M., Mahmoud D.K., Karim W.A.W.A., Idris A. Cationic and Anionic Dye Adsorption by Agricultural Solid Wastes: A Comprehensive Review. Desalination. 2011;280:1–13. doi: 10.1016/j.desal.2011.07.019. - DOI
-
- Zhong Q., Li Y., Zhang G. Two-Dimensional MXene-Based and MXene-Derived Photocatalysts: Recent Developments and Perspectives. Chem. Eng. J. 2021;409:128099. doi: 10.1016/j.cej.2020.128099. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

