Biological Activity of Monoterpene-Based Scaffolds: A Natural Toolbox for Drug Discovery
- PMID: 40286078
- PMCID: PMC11990750
- DOI: 10.3390/molecules30071480
Biological Activity of Monoterpene-Based Scaffolds: A Natural Toolbox for Drug Discovery
Abstract
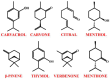
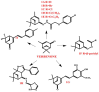
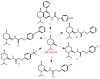
One of the most common strategies used in drug design is the molecular scaffold approach, which combines traditional medicine based on natural active compounds derived from plants with modern synthetic drug development. Designing new compounds based on natural skeletons enables extensive modifications of both bioavailability and biological activity. An excellent example of a natural molecular scaffold is the monoterpenes group, which serves as a core structure for building more complex molecules by attaching various chemical groups. Their ability to interact with biological targets, combined with structural versatility, makes them promising molecular scaffolds in pharmaceutical research and green chemistry applications. This review paper focuses on selected monoterpenes (carvacrol, carvone, citral, menthol, menthone, β-pinene, thymol, and verbenone), which are frequently used as molecular scaffolds. The newly designed derivatives exhibit various biological activities, including anticancer, antibacterial, antiviral, neuroprotective, and many others.
Keywords: carvacrol; carvone; citral; menthol; menthone; molecular scaffold; monoterpenes; thymol; verbenone; β-pinene.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

