Virtual Screening and Bioassay of Novel Protoporphyrinogen Oxidase and p-Hydroxyphenylpyruvate Dioxygenase Dual-Target Inhibitors
- PMID: 40286087
- PMCID: PMC11990379
- DOI: 10.3390/molecules30071491
Virtual Screening and Bioassay of Novel Protoporphyrinogen Oxidase and p-Hydroxyphenylpyruvate Dioxygenase Dual-Target Inhibitors
Abstract
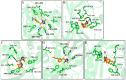
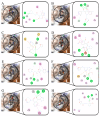
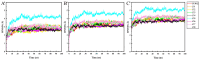
Novel herbicide development is a challenge for weed control. Protoporphyrinogen oxidase (PPO) and p-hydroxyphenylpyruvate dioxygenase (HPPD) are two key enzymes involved in plant photosynthesis. The multi virtual screening protocol was adopted to design a common skeleton based on the two target enzymes, and fragment growth of the skeleton was performed. The constructed compounds were searched for structural similarity, and the accuracy of the selected compounds was further verified using the Bayesian model. Finally, eight compounds were obtained, and the binding mode with the target was studied deeply. The obtained compounds interact with the key residues of HPPD and PPO proteins similarly to commercial herbicides, and the stability of binding with proteins is also good. The activity of the screening results was determined by an enzyme activity test in vitro. The herbicidal effect of the compound was studied by phenotypic experiment. The final results showed that Z-4 and Z-7 have the potential to become new dual-target herbicides.
Keywords: Bayesian model; HPPD; PPO; dual-target herbicide; skeleton-based drug discovery.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










References
-
- Zeng X., Huang Y., Dong J., Ma X., Nan J.X., Chen W., Lin H.Y., Yang W.C., Liu X., Yin J., et al. Design of an HPPD fluorescent probe and visualization of plant responses to abiotic stress. Adv. Agrochem. 2022;1:73–84.
-
- Li L., Gao S., Yang L., Liu Y.L., Li P., Ye F., Fu Y. Cobalt (II) complex as a fluorescent sensing platform for the selective and sensitive detection of triketone HPPD inhibitors. J. Hazard. Mater. 2021;404:124015. - PubMed
-
- Simkin A.J., Kapoor L., Doss C.G.P., Hofmann T.A., Lawson T., Ramamoorthy S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022;152:23–42. - PubMed
-
- Yang Y., Zhou Z., Liu T., Tan Q., Chen L., Wang J., Qiu Z., Ling X., Chen T., Yang X., et al. Multisite Mutagenesis of 4-Hydroxyphenylpyruvate Dioxygenase (HPPD) Enhances Rice Resistance to HPPD Inhibitors and Its Carotenoid Contents. J. Agric. Food Chem. 2024;40:22063–22072. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

