The Role of Flow Chemistry on the Synthesis of Pyrazoles, Pyrazolines and Pyrazole-Fused Scaffolds
- PMID: 40286172
- PMCID: PMC11990259
- DOI: 10.3390/molecules30071582
The Role of Flow Chemistry on the Synthesis of Pyrazoles, Pyrazolines and Pyrazole-Fused Scaffolds
Abstract
Nitrogen-containing heterocycles are fundamental scaffolds in organic chemistry, particularly due to their prevalence in pharmaceuticals, agrochemicals and materials science. Among them, five-membered rings, containing two nitrogen atoms in adjacent positions-such as pyrazoles, pyrazolines and indazoles-are especially significant due to their versatile biological activities and structural properties, which led to the search for greener, faster and more efficient methods for their synthesis. Conventional batch synthesis methods, while effective, often face challenges related to reaction efficiency, scalability and safety. Flow chemistry has emerged as a powerful alternative, offering enhanced control over reaction parameters, improved safety profiles and opportunities for scaling up synthesis processes efficiently. This review explores the impact of flow chemistry on the synthesis of these pivotal heterocycles, highlighting its advantages over the conventional batch methods. Although indazoles have a five-membered ring fused with a benzene ring, they will also be considered in this review due to their biological relevance.
Keywords: flow chemistry; green chemistry; indazoles; pyrazoles; pyrazolines; synthesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





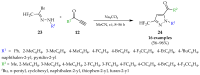



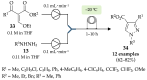





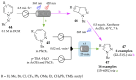
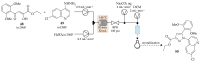













References
-
- Puri S., Sawant S., Juvale K. A Comprehensive Review on the Indazole Based Derivatives as Targeted Anticancer Agents. J. Mol. Struct. 2023;1284:135327. doi: 10.1016/j.molstruc.2023.135327. - DOI
-
- Hassani I.A.E., Rouzi K., Assila H., Karrouchi K., Ansar M. Recent Advances in the Synthesis of Pyrazole Derivatives: A Review. Reactions. 2023;4:478–504. doi: 10.3390/reactions4030029. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

