Impact of mepolizumab on the AAV-PRO questionnaire in eosinophilic granulomatosis with polyangiitis: data from a European multicentre study
- PMID: 40286311
- PMCID: PMC12407232
- DOI: 10.1093/rheumatology/keaf232
Impact of mepolizumab on the AAV-PRO questionnaire in eosinophilic granulomatosis with polyangiitis: data from a European multicentre study
Abstract
Objectives: To prospectively evaluate the impact and the rapidity of the effect of mepolizumab on the ANCA-associated vasculitis patient-reported outcomes (AAV-PRO) questionnaire and patient global assessment (PtGA) in an international, multicentre cohort of patients with eosinophilic granulomatosis with polyangiitis (EGPA).
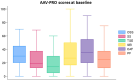
Methods: Patients with active EGPA initiating treatment with mepolizumab were included. PtGA and the AAV-PRO score were assessed at baseline and after 7, 14, 30, 90 and 180 days. Predictors of response of the AAV-PRO questionnaire were investigated.
Results: Seventy patients were included: female 54.3%, median age 56 years (48-65), 63 (90%) with a relapsing/refractory course. PtGA showed a statistically significant decrease within 7 days. At 14 days, all the AAV-PRO domains, except treatment side effects, showed a statistically significant decline. The improvement at 6 months was greatest in organ-specific symptoms (ratio 0.53), physical function (ratio 0.57) and PtGA (ratio 0.58). PtGA and higher disease activity positively correlated with the AAV-PRO scores throughout the study. Female patients reported a greater burden in terms of systemic symptoms, treatment side effects, social and emotional impact and concerns about the future. Conversely, age, educational level, damage accrual, mepolizumab dose and ANCA status had no effect on the AAV-PRO scores.
Conclusions: Mepolizumab was associated with a quick and remarkable improvement of health-related quality of life in patients with EGPA. These findings highlight its early and sustained benefits beyond disease control and support the integration of the AAV-PRO questionnaire into routine clinical practice.
Keywords: ANCA-associated vasculitis patient-reported outcomes questionnaire; eosinophilic granulomatosis with polyangiitis; health-related quality of life; mepolizumab; patient-reported outcomes.
© The Author(s) 2025. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures

References
-
- Jennette JC, Falk RJ, Bacon PA et al. 2012 revised International Chapel Hill Consensus conference nomenclature of vasculitides. Arthritis Rheum 2013;65:1–11. - PubMed
-
- Mahr A, Moosig F, Neumann T et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): evolutions in classification, etiopathogenesis, assessment and management. Curr Opin Rheumatol 2014;26:16–23. - PubMed
-
- Comarmond C, Pagnoux C, Khellaf M et al. ; French Vasculitis Study Group. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum 2013;65:270–81. - PubMed
-
- Bettiol A, Sinico RA, Schiavon F et al. ; Italian EGPA Consortium. Risk of acute arterial and venous thromboembolic events in eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome). Eur Respir J 2021;57:2004158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

