A Comparison of Outcomes With Apixaban, Rivaroxaban, and Warfarin for Atrial Fibrillation and/or Venous Thromboembolism
- PMID: 40286370
- PMCID: PMC12235406
- DOI: 10.1016/j.jacadv.2025.101714
A Comparison of Outcomes With Apixaban, Rivaroxaban, and Warfarin for Atrial Fibrillation and/or Venous Thromboembolism
Abstract
Background: Apixaban and rivaroxaban are commonly used direct oral anticoagulants for atrial fibrillation (AF) and venous thromboembolism (VTE). Both have been compared to warfarin, but there are insufficient comparative outcome data.
Objectives: The purpose of this study was to assess outcomes of apixaban, rivaroxaban, and warfarin.
Methods: This is a registry-based cohort study with data from 6 centers in Michigan, 2009 to 2023. Patients were adults with AF and/or VTE with at least 3 months of follow-up. Outcomes included rates of bleeding, thrombosis, healthcare utilization, and death.
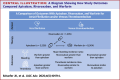
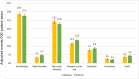
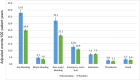
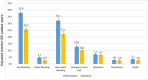
Results: A total of 13,435 patients met the study inclusion criteria (average age 66.7 years, 58.0% on anticoagulation for AF, average follow-up 28.2 months). After matching, 2 groups of 3,527 patients on apixaban and warfarin were compared. Any bleeding was similar between groups, but major bleeding was less with apixaban. Thrombotic event rates were higher with apixaban. Mortality, rates of emergency room visits, and hospitalizations for bleeding were higher with warfarin. After matching, 1,395 patients on rivaroxaban were compared to 4,185 patients on warfarin. Any bleeding and major bleeding were higher with rivaroxaban. Thrombotic event rates were similar, aside from a higher rate of "other" thrombosis with rivaroxaban. After matching, 2 groups of 1,395 patients on apixaban and rivaroxaban were compared. Any bleeding, major bleeding, and mortality were higher with rivaroxaban. Thrombotic event rates were similar.
Conclusions: For patients with AF and/or VTE, we observed that bleeding was highest with rivaroxaban, followed by warfarin, and then apixaban. Rates of thrombosis were higher with apixaban than with warfarin, largely driven by "other" thrombotic events.
Keywords: atrial fibrillation; factor Xa inhibitors; hemorrhage; outcomes assessment; venous thromboembolism; warfarin.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding support and author disclosures Support for the Michigan Anticoagulation Quality Improvement Initiative is provided by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of the BCBSM Value Partnerships program. Although Blue Cross Blue Shield of Michigan and the Michigan Anticoagulation Quality Improvement Initiative work collaboratively, the opinions, beliefs, and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation review or approval of the manuscript; and decision to submit the manuscript for publication. Dr Schaefer has received grants from the American Society of Hematology, a HTRS Mentored Research Award, which was supported by an educational grant from Takeda; and is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL169920. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health; has received personal fees from Pfizer and Sanofi. Dr Kaatz has received grants from Osmosis Research, Janssen, Bristol Myers Squibb, Bayer, and the National Institutes of Health; personal fees from Janssen, Bristol Myers Squibb, Pfizer, Gilead, PhaseBio, Astra Zeneca, Inari, Antos, and Boston Scientific; is on the Board of Directors for Anticoagulation Forum and the PERT Consortium; and is on the Scientific Advisory Board for the National Blood Clot Alliance. Dr Froehlich has received grant funding from Blue Cross Blue Shield of Michigan, the Fibromuscular Disease Society of America, and the PERT Consortium; and serves as a Committee Chair for the American Heart Association. Dr Barnes has received grant funding from Blue Cross Blue Shield of Michigan, the National Institutes of Health, and Boston Scientific; has received personal fees from Pfizer/Bristol Myers Squibb, Janssen, Abbott Vascular, Boston Scientific, Bayer, Sanofi, and Anthos; and is on the Board of Directors for the Anticoagulation Forum. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
References
-
- Joglar J.A., Chung M.K., Armbruster A.L., et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2024;149(1):e1–e156. doi: 10.1161/CIR.0000000000001193. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

