Taurine ameliorates cellular senescence associated with an increased hydrogen sulfide and a decreased hepatokine, IGFBP-1, in CCl4-induced hepatotoxicity in mice
- PMID: 40286436
- PMCID: PMC12059708
- DOI: 10.1016/j.redox.2025.103640
Taurine ameliorates cellular senescence associated with an increased hydrogen sulfide and a decreased hepatokine, IGFBP-1, in CCl4-induced hepatotoxicity in mice
Abstract
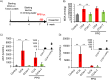
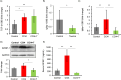
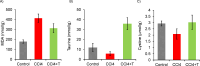
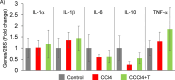
This study investigated the protective effects of taurine against cellular senescence and hepatokine secretion in a mouse model of carbon tetrachloride (CCl4)-induced chronic liver injury. Oral taurine administration by tap water containing 3 % taurine significantly attenuated liver damage, as evidenced by reduced serum AST, ALT level and hepatic lipid peroxidation. Importantly, hepatic taurine level is reduced in CCl4-induced injury model, while taurine administration recovered it. Moreover, taurine administration decreased the numbers of p21-positive senescent cells in liver tissue of CCl4-treated mice. Taurine increases hydrogen sulfide (H2S) in liver of normal mice, suggesting anti-oxidative role through H2S production by taurine. Furthermore, inhibition of CTH, which is an enzyme responsible for H2S production from cysteine, by propagylglycine attenuated malondialdehyde-lowering effect of taurine in liver of CCl4-treated mice. Moreover, we found taurine treatment lowers insulin-like growth factor binding protein-1 (IGFBP-1) in liver of normal mice. Importantly, while chronic CCl4 injection caused an induction of IGFBP-1, taurine administration blocked it. These findings suggest that taurine exerts its protective effects by attenuating cellular senescence, which is associated with enhancing H2S production and inhibiting IGFBP-1 expression. This study highlights the potential of taurine as a therapeutic strategy for mitigating chronic liver injury by producing H2S and targeting IGFBP1.
Keywords: Hepatokines; Hydrogen sulfide; IGFBP-1; Liver injury; Senescence; Taurine.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Ogrodnik M., Carlos Acosta J., Adams P.D., d'Adda di Fagagna F., Baker D.J., Bishop C.L., Chandra T., Collado M., Gil J., Gorgoulis V., Gruber F., Hara E., Jansen-Dürr P., Jurk D., Khosla S., Kirkland J.L., Krizhanovsky V., Minamino T., Niedernhofer L.J., Passos J.F., Ring N.A.R., Redl H., Robbins P.D., Rodier F., Scharffetter-Kochanek K., Sedivy J.M., Sikora E., Witwer K., von Zglinicki T., Yun M.H., Grillari J., Demaria M. Guidelines for minimal information on cellular senescence experimentation in vivo. Cell. 2024;187:4150–4175. doi: 10.1016/J.CELL.2024.05.059. - DOI - PMC - PubMed
-
- Kiourtis C., Terradas-Terradas M., Gee L.M., May S., Georgakopoulou A., Collins A.L., O'Sullivan E.D., Baird D.P., Hassan M., Shaw R., Tan E.H., Müller M., Engelmann C., Andreola F., Hsieh Y.C., Reed L.H., Borthwick L.A., Nixon C., Clark W., Hanson P.S., Sumpton D., Mackay G., Suzuki T., Najumudeen A.K., Inman G.J., Campbell A., Barry S.T., Quaglia A., Morris C.M., LeBeau F.E.N., Sansom O.J., Kirschner K., Jalan R., Oakley F., Bird T.G. Hepatocellular senescence induces multi-organ senescence and dysfunction via TGFβ. Nat. Cell Biol. 2024. 2024;2612 26:2075–2083. doi: 10.1038/s41556-024-01543-3. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

