Phase separation as a key mechanism in plant development, environmental adaptation, and abiotic stress response
- PMID: 40286852
- PMCID: PMC12173016
- DOI: 10.1016/j.jbc.2025.108548
Phase separation as a key mechanism in plant development, environmental adaptation, and abiotic stress response
Abstract
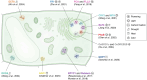
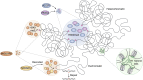
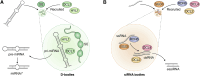
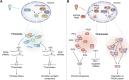
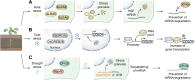
In memoriam of Professor Anderson de Sá Pinheiro, principal investigator at the Laboratory of Molecular Biology (LabMol) at the Institute of Chemistry, Federal University of Rio de Janeiro (UFRJ). Prof. Pinheiro passed away prematurely at the age of 44, on March 1, 2025. Prof. Pinheiro was a distinguished figure in the fields of biochemistry and structural biology in Brazil. He earned his bachelor's degree in Pharmacy in 2000, his master's degree in 2003, and his Ph.D. in 2007, all in Biological Chemistry at UFRJ. He continued his academic journey with postdoctoral research at Brown University in the United States (2007-2011). Upon returning to Brazil, he became an Associate Professor in the Department of Biochemistry at UFRJ (2011-2025). He led the Laboratory of Molecular Biochemistry (LaBMol), focusing on the study of RNA-binding proteins related to cancer and neurodegenerative disorders, as well as plant responses to abiotic stress. His research followed two main fronts-analyzing protein structures and dynamics using solution NMR spectroscopy and investigating the relationship between structural features and liquid-liquid phase separation, along with its role in protein function. Beyond research, Prof. Anderson was deeply committed to education, mentoring numerous students and contributing to various academic committees. During his brief but impactful career, he made significant contributions to the structural biology community, serving as President of the Brazilian Association of Nuclear Magnetic Resonance Users (AUREMN) and as Scientific Director of the Brazilian Biophysical Society (SBBf). This review marks Professor Pinheiro's 50th published article. His untimely passing is a profound loss to the scientific community, but his legacy endures through his scientific contributions and the many lives he has touched. Liquid-liquid phase separation is a fundamental biophysical process in which biopolymers, such as proteins, nucleic acids, and their complexes, spontaneously demix into distinct coexisting phases. This phenomenon drives the formation of membraneless organelles-cellular subcompartments without a lipid bilayer that perform specialized functions. In plants, phase-separated biomolecular condensates play pivotal roles in regulating gene expression, from genome organization to transcriptional and post-transcriptional processes. In addition, phase separation governs plant-specific traits, such as flowering and photosynthesis. As sessile organisms, plants have evolved to leverage phase separation for rapid sensing and response to environmental fluctuations and stress conditions. Recent studies highlight the critical role of phase separation in plant adaptation, particularly in response to abiotic stress. This review compiles the latest research on biomolecular condensates in plant biology, providing examples of their diverse functions in development, environmental adaptation, and stress responses. We propose that phase separation represents a conserved and dynamic mechanism enabling plants to adapt efficiently to ever-changing environmental conditions. Deciphering the molecular mechanisms underlying phase separation in plant stress responses opens new avenues for biotechnological strategies aimed at engineering stress-resistant crops. These advancements have significant implications for agriculture, particularly in addressing crop productivity in the face of climate change.
Keywords: condensate; growth; phase; plant; separation; stress.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Shin Y., Brangwynne C.P. Liquid phase condensation in cell physiology and disease. Science. 2017;357 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

