Parkinson's disease is characterized by vitamin B6-dependent inflammatory kynurenine pathway dysfunction
- PMID: 40287426
- PMCID: PMC12033312
- DOI: 10.1038/s41531-025-00964-7
Parkinson's disease is characterized by vitamin B6-dependent inflammatory kynurenine pathway dysfunction
Abstract
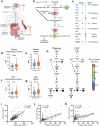
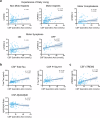
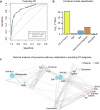
Recent studies demonstrate that Parkinson's disease (PD) is associated with dysregulated metabolic flux through the kynurenine pathway (KP), in which tryptophan is converted to kynurenine (KYN), and KYN is subsequently metabolized to neuroactive compounds quinolinic acid (QA) and kynurenic acid (KA). Here, we used mass-spectrometry to compare blood and cerebral spinal fluid (CSF) KP metabolites between 158 unimpaired older adults and 177 participants with PD. We found increased neuroexcitatory QA/KA ratio in both plasma and CSF of PD participants associated with peripheral and cerebral inflammation and vitamin B6 deficiency. Furthermore, increased QA tracked with CSF tau, CSF soluble TREM2 (sTREM2) and severity of both motor and non-motor PD clinical symptoms. Finally, PD patient subgroups with distinct KP profiles displayed distinct PD clinical features. These data validate the KP as a site of brain and periphery crosstalk, integrating B-vitamin status, inflammation and metabolism to ultimately influence PD clinical manifestation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Update of
-
Parkinson's disease is characterized by vitamin B6-dependent inflammatory kynurenine pathway dysfunction.Res Sq [Preprint]. 2024 Sep 26:rs.3.rs-4980210. doi: 10.21203/rs.3.rs-4980210/v1. Res Sq. 2024. Update in: NPJ Parkinsons Dis. 2025 Apr 26;11(1):96. doi: 10.1038/s41531-025-00964-7. PMID: 39399688 Free PMC article. Updated. Preprint.
References
-
- Poewe, W. et al. Parkinson disease. Nat. Rev. Dis. Prim.3, 17013 (2017). - PubMed
-
- Tan, E. K. et al. Parkinson disease and the immune system - associations, mechanisms and therapeutics. Nat. Rev. Neurol.16, 303–318 (2020). - PubMed
-
- Harms, A. S., Yang, Y. T. & Tansey, M. G. Central and peripheral innate and adaptive immunity in Parkinson’s disease. Sci. Transl. Med15, eadk3225 (2023). - PubMed
Grants and funding
- R01AG074339/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01AG048232/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- 6440.0/Michael J. Fox Foundation for Parkinson's Research (Michael J. Fox Foundation)
- R35GM138353/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- U24 AG21886/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P30 ES013508/ES/NIEHS NIH HHS/United States
- RF1AG058047/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P30 AG066518/AG/NIA NIH HHS/United States
- R37 NS115139/NS/NINDS NIH HHS/United States
- P30 AG066515/AG/NIA NIH HHS/United States
- R01AG048076/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- RO1NS115139/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P01 AG084497/AG/NIA NIH HHS/United States
- P50NS062684/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- P30 AG072979/AG/NIA NIH HHS/United States
- 19PABHI34580007/American Heart Association (American Heart Association, Inc.)
- K23NS075097/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P30AG066518/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P30AG066515/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01NS115114/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources

