Subthalamic stimulation evokes hyperdirect high beta interruption and cortical high gamma entrainment in Parkinson's disease
- PMID: 40287435
- PMCID: PMC12033315
- DOI: 10.1038/s41531-025-00965-6
Subthalamic stimulation evokes hyperdirect high beta interruption and cortical high gamma entrainment in Parkinson's disease
Abstract
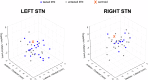
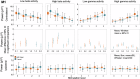
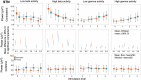
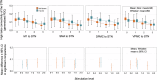
Compound network dynamics in beta and gamma bands determine the severity of bradykinesia in Parkinson's disease. We explored its subthalamic stimulation related changes parallel with improvement of complex hand movements. Thirty eight patients with Parkinson's disease treated with bilateral stimulation accomplished voluntary and traced spiral drawing with their more affected hand on a digital tablet. A 64 channel electroencephalography was recorded, low and high beta and gamma power was computed in subthalamic and motor cortical sources at four stimulation levels. Subthalamic cortical effective connectivity was calculated, and subnetwork models were created. Beta power decreased, and gamma power increased in sources ipsilateral to stimulation with increasing stimulation intensity. Networks comprising the primary motor cortex played a dominant role in predicting the improvement of voluntary drawing speed. Subthalamic stimulation diminished the hyperdirect high beta information processing and promoted the cortico cortical interactions of the primary motor cortex in the high gamma band.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.M. is the Deputy Editor-in-chief of this journal. Other authors have no competing interest to declare.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

