Recombinant production of spider silk protein in Physcomitrella photobioreactors
- PMID: 40287554
- PMCID: PMC12033203
- DOI: 10.1007/s00299-025-03485-y
Recombinant production of spider silk protein in Physcomitrella photobioreactors
Abstract
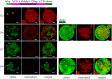
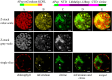
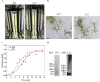
We report the successful moss-produced recombinant spider silk key protein component containing both the N- and the C-terminal domain. Spider dragline silk stands out as a remarkable biomaterial, representing one of nature's toughest fibres. Its strength rivals that of many synthetic fibres used commercially, rendering it applicable across various industrial and medical domains. However, its widespread utilisation requires cost-effective mass production. Biotechnology presents a promising avenue for achieving this goal, particularly through the production of recombinant dragline silk proteins in transgenic plant systems. This study aimed to assess the feasibility of producing one key protein component of dragline silk, MaSp1, from the western black widow spider, Latrodectus hesperus, the protein LhMaSp1, in the moss Physcomitrella (Physcomitrium patens). Here, we present the successful recombinant production of spider silk protein containing both the N- and C-terminal domains of LhMaSp1 in moss cells. The production of recombinant LhMaSp1 protein in Physcomitrella was performed in shake flasks and in five-litre photobioreactors and the correct synthesis of LhMaSp1 was proven via mass spectrometry. We estimate that the yield of recombinant spider silk protein in Physcomitrella bioreactors is above 0.82 mg/g fresh weight.
Keywords: Bioreactor; Bryotechnology; Dragline silk protein; Plant-made protein; Smart materials; Spidroins.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: All authors declare no conflict of interest.
Figures


Similar articles
-
Complex gene expression in the dragline silk producing glands of the Western black widow (Latrodectus hesperus).BMC Genomics. 2013 Dec 2;14:846. doi: 10.1186/1471-2164-14-846. BMC Genomics. 2013. PMID: 24295234 Free PMC article.
-
Spider silks from plants - a challenge to create native-sized spidroins.Biotechnol J. 2013 Oct;8(10):1183-92. doi: 10.1002/biot.201300204. Biotechnol J. 2013. PMID: 24092675 Review.
-
Expression of spider silk protein in tobacco improves drought tolerance with minimal effects on its mechanotype.Plant J. 2025 Jan;121(2):e17213. doi: 10.1111/tpj.17213. Plant J. 2025. PMID: 39866095 Free PMC article.
-
Low-Tech, Pilot Scale Purification of a Recombinant Spider Silk Protein Analog from Tobacco Leaves.Int J Mol Sci. 2016 Oct 9;17(10):1687. doi: 10.3390/ijms17101687. Int J Mol Sci. 2016. PMID: 27735843 Free PMC article.
-
Spider silks and their applications.Trends Biotechnol. 2008 May;26(5):244-51. doi: 10.1016/j.tibtech.2008.02.006. Epub 2008 Mar 25. Trends Biotechnol. 2008. PMID: 18367277 Review.
References
-
- Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL (2003) Silk-based biomaterials. Biomaterials 24:401–416. 10.1016/S0142-9612(02)00353-8 - PubMed
-
- Andresen PA, Kaasen I, Styrvold OB, Boulnois GJ, Strøm AR (1988) Molecular cloning, physical mapping and expression of the bet genes governing the osmoregulatory choline-glycine betaine pathway of Escherichia coli. J Gen Microbiol 134:1737–1746. 10.1099/00221287-134-6-1737 - PubMed
-
- Anton AM, Heidebrecht A, Mahmood N, Beiner M, Scheibel T, Kremer F (2017) Foundation of the outstanding toughness in biomimetic and natural spider silk. Biomacromol 18:3954–3962. 10.1021/acs.biomac.7b00990 - PubMed
-
- Askarieh G, Hedhammar M, Nordling K, Saenz A, Casals C, Rising A, Johansson J, Knight SD (2010) Self-assembly of spider silk proteins is controlled by a pH-sensitive relay. Nature 465:236–238. 10.1038/nature08962 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

