Bioequivalence and Safety of Two Amisulpride Formulations in Healthy Chinese Subjects Under Fasting and Fed Conditions: A Randomized, Open‑Label, Single‑Dose, Crossover Study
- PMID: 40287564
- PMCID: PMC12185791
- DOI: 10.1007/s40268-025-00508-7
Bioequivalence and Safety of Two Amisulpride Formulations in Healthy Chinese Subjects Under Fasting and Fed Conditions: A Randomized, Open‑Label, Single‑Dose, Crossover Study
Abstract
Background and objectives: Amisulpride is a second-generation antipsychotic drug that selectively binds to D2 and D3 dopaminergic receptors in the limbic system. In this study, the bioequivalence of an amisulpride formulation manufactured in China with the original formulation Solian® was evaluated under fasting and fed conditions in healthy Chinese subjects.
Methods: A single-centre, randomized, open, two-preparation, single-dose, two-period crossover trial in healthy adult subjects was conducted under fasting and fed conditions. A total of 42 and 36 eligible healthy subjects were enrolled in the fasting and fed studies, respectively. The subjects were randomly assigned to receive either the test or the reference formulation with a washout period of 7 days. The concentration of amisulpride in plasma was determined by liquid chromatography‒tandem mass spectrometry (LC‒MS/MS), and the pharmacokinetic (PK) parameters of amisulpride were calculated via the noncompartmental method.
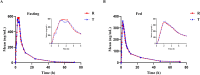
Results: The geometric mean ratios (GMR) of the maximum observed concentration (Cmax), the area under the plasma concentration‒time curve (AUC) from time zero to the last sampling time (AUC0-t), and the AUC from time zero to infinity (AUC0-∞) from the test/reference formulation under fasting conditions were 93.83, 101.90, and 102.35, respectively, with corresponding 90% confidence intervals (CIs) of 83.93-104.89, 97.58-106.42, and 98.24-106.63. The GMRs of Cmax, AUC0-t, and AUC0-∞ under fed conditions were 102.23, 106.09, and 101.87, respectively, with corresponding 90% CIs of 92.49-112.99, 102.44-109.87, and 97.49-106.44. These data all satisfied the bioequivalence criteria (90% CIs in the range of 80-125%). In terms of safety, no serious adverse events were observed.
Conclusions: The test and reference amisulpride formulations were bioequivalent under fasting and fed conditions. Both formulations showed similar safety and tolerability in the population studied.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of Interests: The authors declare no conflicts of interest regarding the content of this article. Funding Statement: This study was sponsored and funded by Fujian Baonuo Pharmaceutical R&D Co. Ethics Approval: All the protocol and documents were reviewed and approved by the Ethics Committee of Wuhan Pulmonary Hospital (no. 2021005). This study was registered at the Drug Clinical Trial Registration and Information Publicity Platform ( http://www.chinadrugtrials.org.cn/index.html ) with registration no. CTR20211003. Consent to Participate: All study participants provided written informed consent. Consent for Publication: All researchers, participants, institutions and sponsors consented to the submission of this report to the journal. Data Availability Statement: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Code Availability: Not applicable. Author Contributions: W.M. took responsibility for writing the manuscript and contributed to the pharmacokinetic analysis as well as data interpretation. P.Y. was responsible for the management of drugs in the study and manuscript submission. Y.H.G. participated in quality control throughout the study. P.Z.X. was involved in the management of biological samples. D.R.H. and L.G. were responsible for the conception and design of the study, data analysis and revision of the manuscript. All the authors have read and approved the final manuscript.
Figures
References
-
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease study 2019. Lancet Psychiatry. 2022;9(2):137–50. 10.1016/S2215-0366(21)00395-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials