Calciprotein particle-activated endothelial cells aggravate smooth muscle cell calcification via paracrine signalling
- PMID: 40287595
- PMCID: PMC12033162
- DOI: 10.1007/s00018-025-05702-z
Calciprotein particle-activated endothelial cells aggravate smooth muscle cell calcification via paracrine signalling
Abstract
Background: Vascular calcification is highly prevalent in Chronic Kidney Disease (CKD) and is associated with markedly increased cardiovascular risk. High serum phosphate in CKD increases calcification propensity via generation of circulating calciprotein particles (CPP2), crystalline nanoaggregates composed of calcium, phosphate, and serum proteins. CPP2 induce vascular calcification in vascular smooth muscle cells (VSMCs) in vitro. In vivo, endothelial cells, rather than VSMCs are primarily exposed to CPP2, yet understanding the influence of endothelial cells on vascular calcification is limited.
Methods: We investigated calcification-promoting signalling by endothelial cells on VSMCs. Effects of CPP2 exposure to endothelial cells on CPP2 uptake, endothelial cell activation, and endothelial cell-derived secretome were studied. Effects of the secretome on VSMC calcification were investigated. Using NanoString nCounter analysis the effects of CPP2-activated endothelial cell-conditioned medium on VSMCs gene expression were mapped.
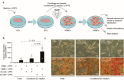
Results: Endothelial cells internalise CPP2 and elevate ICAM-1, E-selectin, and VCAM-1-mRNA expression, indicating endothelial activation. VSMCs cultured in conditioned medium from CPP2-activated endothelial cells demonstrated enhanced calcification, suggesting that CPP2-activated endothelial cells release pro-calcifying soluble factors. Mass spectrometry was utilized to identify 1171 proteins in the CPP2-activated endothelial cells' secretome. Among these, 76 proteins were differentially expressed compared to control endothelial cells' secretome, including proteins related to blood vessel development, extracellular matrix remodelling, and oxidative stress-related processes. Finally, endothelial cell-derived paracrine factors present in conditioned medium enhanced mRNA-expression of calcification-related factors in VSMCs.
Conclusions: CPP2-activated endothelial cells promote VSMC calcification via paracrine signalling. In response to these paracrine factors, VSMCs increase the expression of pro-calcification genes.
Keywords: Calciprotein particles; Chronic kidney disease; Endothelial cell activation; Paracrine signalling; Vascular calcification.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: G. Krenning is Chief Scientific Officer of Sulfateq B.V. (Groningen, the Netherlands), a company that develops small molecule therapeutics. Sulfateq B.V. has no small molecule in development for anti-circulating calciprotein particle (CPP2) therapy at present and had no influence on the content of this paper. E.R. Smith is a stockholder and former scientific advisor of Calciscon AG and has also received honoraria from CSL Vifor. Funding from Astellas received by R.A. Pol had no influence on the content of the paper. Astellas was not involved in the design of the study, collection of data, analysis and interpretation of data, management, manuscript preparation or the decision to submit the paper for publication. All other authors report no conflict of interest. Ethics approval: The study was conducted in compliance with the principle of the Declaration of Helsinki. All patients gave written informed consent. The protocol was reviewed and approved by the Institutional Review Board of the UMCG (number 2014–077).
Figures





References
-
- Schwarz U, Buzello M, Ritz E et al (2000) Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant 15:218–223. 10.1093/ndt/15.2.218 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

