Viral activity in lake analogs of anoxic early Earth oceans
- PMID: 40287716
- PMCID: PMC12032784
- DOI: 10.1186/s40168-025-02085-y
Viral activity in lake analogs of anoxic early Earth oceans
Abstract
Background: Meromictic lakes, with their stratified water columns, are modern analogs for ancient euxinic (anoxic and sulfidic) oceans, where anaerobic sulfur-oxidizing purple and green sulfur bacteria (PSB and GSB) dominated as primary producers. Recent studies suggest a potential role of viruses in the metabolisms and biosignatures of these bacteria, but conclusive evidence of viral replication and activity in such lakes is still lacking.
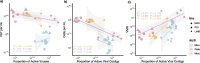
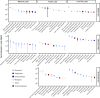
Results: Here, we investigate viral activity in the upper mixed layer (mixolimnion), the anoxic bottom (monimolimnion), and the microbial plate (a dense layer of phototrophic sulfur bacteria forming at the boundary between the oxygenated mixolimnion and the anoxic monimolimnion) of three meromictic lakes: Poison and Lime Blue Lakes (WA, USA) and Mahoney Lake (BC, CA). Geochemical profiles of two lakes, Mahoney and Poison, which are dominated by PSB, show a sharp chemocline, whereas Lime Blue displays a less steep chemical gradient and hosts a mixture of PSB and GSB. Viral gene transcription and epifluorescence microscopy revealed depth-dependent patterns in viral activity. The two strongly stratified, PSB-dominated lakes showed a significant decrease in the virus-to-microbe ratio (VMR) in their microbial plates, suggesting reduced viral particle production via lysis. Metatranscriptome data corroborated this trend by showing lower levels of viral gene expression in these microbial plates, higher expression of CRISPR defense and lysogeny-related genes, and relatively high expression of photosynthesis-related viral genes. Conversely, the third lake, which harbors a mix of PSB and GSB, exhibited low microbial density, high VMR, and high viral transcriptional activity. Viral transcription levels significantly correlated with VMR in the microbial plates and bottom layers, but this relationship was absent in low-density, oxic surface samples.
Conclusions: Here, two independent lines of evidence, abundances and gene expression, show reduced viral lytic production in microbial plates dominated by PSB in stratified lakes. This suggests that viral lysis may contribute less to bacterial community structuring in these high-density microbial plates. Rather, other viral-mediated mechanisms, such as lysogeny and the expression of auxiliary metabolic genes, may represent a more significant viral influence on bacterial physiology and geochemistry. These patterns in virus-bacteria interactions may be consequential for the interpretations of biosignatures left by these bacterial groups in the geologic record. Video Abstract.
Keywords: Bacteriophage; Meromictic lake; Viral activity; Viral metatranscriptomics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Xiong Y, Guilbaud R, Peacock CL, Cox RP, Canfield DE, Krom MD, et al. Phosphorus cycling in Lake Cadagno, Switzerland: a low sulfate euxinic ocean analogue. Geochimica et Cosmochimica Acta. 2019;251:116–35.
-
- Overmann J, Beatty JT, Krouse HR, Hall KJ. The sulfur cycle in the chemocline of a meromictic salt lake. Limnol Oceanogr. 1996;41(1):147–56.
-
- Overmann J, Beatty JT, Hall KJ, Pfennig N, Northcote TG. Characterization of a dense, purple sulfur bacterial layer in a meromictic salt lake. Limnol Oceanogr. 1991;36(5):846–59.
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

