Cardiomyocyte-specific NHE1 overexpression confers protection against myocardial infarction during hyperglycemia
- PMID: 40287728
- PMCID: PMC12034198
- DOI: 10.1186/s12933-025-02743-3
Cardiomyocyte-specific NHE1 overexpression confers protection against myocardial infarction during hyperglycemia
Abstract
Background: Acute hyperglycemia on admission is frequently observed during the early phase after acute myocardial infarction (MI), even without the history of diabetes mellitus. We previously reported that inhibiting Na+/H+ exchanger 1 (NHE1) activity post-MI may improve outcomes, but not in the setting of MI with acute hyperglycemia. However, the precise role of NHE1 in the pathophysiology of MI with acute hyperglycemia remains to be elucidated, and there are no effective strategies for its prevention or treatment.
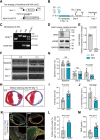
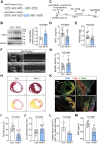
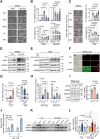
Methods and results: We analyzed 85 post-MI patients, identifying acute hyperglycemia (glucose > 7 mM) in non-diabetic individuals, linked to elevated BNP, CK-MB, and reduced plasma Na+. Using retrospective cohort studies and MI with acute hyperglycemia mouse models, we demonstrated that hyperglycemia exacerbates myocardial injury by reducing extracellular Na+, increasing intracellular Na+, and elevating pH, suggesting NHE1 activation as inferred from the observed intracellular pH (pHi) shift. Cardiomyocyte-specific NHE1 ablation or pharmacological inhibition worsened cardiac dysfunction and fibrosis in MI with acute hyperglycemia, while NHE1 overexpression conferred protection. RNA sequencing and drug screening identified accelerated NHE1 activation via 3% NaCl and lithospermic acid (LA) as a novel strategy to mitigate cardiomyocyte necroptosis, alleviating ischemic injury in MI and ischemia reperfusion models. Hypoxia-hyperglycemia and necroptosis induction models in NHE1-knockout, NHE1-overexpressing, and MLKL-overexpressing cardiomyocytes revealed that NHE1 activation, unlike its protective role in oxygen-glucose deprivation, promotes MLKL degradation via autophagosome-lysosomal pathways, reducing cardiomyocyte death. MLKL knockout and MLKL-NHE1 double knockout mice confirmed that MLKL ablation counteracts NHE1 inhibition's detrimental effects.
Conclusions: Activation of myocardial NHE1 promotes MLKL autophagic degradation, mitigating cardiomyocyte necroptosis and acute hyperglycemia-exacerbated MI, highlighting NHE1 as a hyperglycemia-dependent cardioprotective target. Moderate NHE1 activation may represent a novel therapeutic strategy for MI with acute hyperglycemia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Fibronectin type III domain containing 4 alleviates myocardial ischemia/reperfusion injury via the Nrf2-dependent antioxidant pathway.Free Radic Biol Med. 2024 Nov 1;224:256-271. doi: 10.1016/j.freeradbiomed.2024.08.033. Epub 2024 Aug 26. Free Radic Biol Med. 2024. PMID: 39197598
-
Off-target effects of sodium-glucose co-transporter 2 blockers: empagliflozin does not inhibit Na+/H+ exchanger-1 or lower [Na+]i in the heart.Cardiovasc Res. 2021 Dec 17;117(14):2794-2806. doi: 10.1093/cvr/cvaa323. Cardiovasc Res. 2021. PMID: 33135077 Free PMC article.
-
Empagliflozin prevents heart failure through inhibition of the NHE1-NO pathway, independent of SGLT2.Basic Res Cardiol. 2024 Oct;119(5):751-772. doi: 10.1007/s00395-024-01067-9. Epub 2024 Jul 24. Basic Res Cardiol. 2024. PMID: 39046464 Free PMC article.
-
The myocardial Na+/H+ exchanger: a potential therapeutic target for the prevention of myocardial ischaemic and reperfusion injury and attenuation of postinfarction heart failure.Drugs. 2001;61(3):375-89. doi: 10.2165/00003495-200161030-00006. Drugs. 2001. PMID: 11293648 Review.
-
Regulated cell death in acute myocardial infarction: Molecular mechanisms and therapeutic implications.Ageing Res Rev. 2025 Feb;104:102629. doi: 10.1016/j.arr.2024.102629. Epub 2024 Dec 5. Ageing Res Rev. 2025. PMID: 39644925 Review.
References
-
- Deedwania P, Kosiborod M, Barrett E, Ceriello A, Isley W, Mazzone T, Raskin P. Hyperglycemia and acute coronary syndrome: a scientific statement from the American heart association diabetes committee of the council on nutrition, physical activity, and metabolism. Circulation. 2008;117(12):1610–9. - PubMed
-
- Zarich SW, Nesto RW. Implications and treatment of acute hyperglycemia in the setting of acute myocardial infarction. Circulation. 2007;115(18):e436-439. - PubMed
-
- Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, Eesa M, Fischer U, Hausegger K, Hirsch JA, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke Off J Int Stroke Soc. 2018;13(6):612–32. - PubMed
-
- Yang J, Zheng Y, Li C, Gao J, Meng X, Zhang K, Wang W, Shao C, Tang YD. The impact of the stress hyperglycemia ratio on short-term and long-term poor prognosis in patients with acute coronary syndrome: insight from a large cohort study in Asia. Diabetes Care. 2022;45(4):947–56. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

