The role of tenofovir-based HIV pre-exposure prophylaxis in preventing HBV infection among men who have sex with men: insights from China
- PMID: 40287745
- PMCID: PMC12034112
- DOI: 10.1186/s40249-025-01305-9
The role of tenofovir-based HIV pre-exposure prophylaxis in preventing HBV infection among men who have sex with men: insights from China
Abstract
Background: Oral emtricitabine-tenofovir disoproxil fumarate (F/TDF) for HIV pre-exposure prophylaxis (PrEP) demonstrates dual potential through antiviral activity against hepatitis B virus (HBV). While F/TDF lacks activity against hepatitis C virus (HCV), the use of F/TDF for HIV PrEP may elevate HCV risk through risk compensation. This study aims to investigate HBV/HCV incidence among men who have sex with men (MSM) using F/TDF-based HIV PrEP, addressing evidence gaps in low- and middle-income countries.
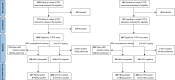
Methods: We conducted a secondary analysis of the China Real-World Oral Intake of PrEP (CROPrEP) study, a multicenter prospective cohort of MSM (F/TDF users/non-users) from Beijing, Shenyang, Shenzhen, and Chongqing. Participants underwent HBV/HCV testing at baseline and at the 12-month follow-up. Only HBV-susceptible (hepatitis B surface antigen-negative, hepatitis B surface and core antibody-negative) MSM were included in the secondary analysis, to calculate HBV incidence. The primary outcomes were HBV/HCV incidence rates at the 12-month follow-up. Bayesian Poisson regression identified HBV/HCV infection risk factors.
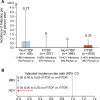
Results: The CROPrEP cohort prospectively recruited 1023 F/TDF users and 507 F/TDF non-users at baseline. This secondary analysis included 259 F/TDF users and 120 non-users identified as HBV-susceptible at baseline. At the 12-month of follow-up, no incident HBV infections occurred in the F/TDF users group, and only one incident HBV infection occurred in the F/TDF non-users group. The incidence of new HBV infections was 0.00/100 person-years (PY) [95% confidence interval (CI): 0.00-1.32] among HBV-susceptible F/TDF users and 0.77/100 PY (95% CI: 0.02-4.20) among HBV-susceptible F/TDF non-users. HBV incidence was reduced with F/TDF compared with no F/TDF [adjusted incidence rate ratio (aIRR): 0.00; 95% CI: 0.00-0.00]. HCV incidence among F/TDF users and non-users was 0.31/100 PY (95% CI: 0.06-0.90) and 0.00/100 PY (95% CI: 0.00-0.74) after 12 months, respectively. HCV incidence was lower in F/TDF non-users than in F/TDF users (aIRR: 0.00; 95% CI: 0.00-0.25).
Conclusions: This study suggests a potential benefit in reducing HBV incidence among MSM using F/TDF as HIV PrEP, highlighting the potential for integrated prevention strategies addressing both HIV and HBV risks in PrEP programmes.
Trial registration: ChiCTR, ChiCTR-IIN-17013762. Registered 8 December 2017, https://www.chictr.org.cn/showproj.html?proj=22916 .
Keywords: Bayesian Poisson regression; Hepatitis B virus; Hepatitis C virus; Incidence; Men who have sex with men; Pre-exposure prophylaxis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study’s protocol was approved by the Ethics Review Committee of the First Affiliated Hospital of China Medical University ([2018]2015-139-5). All participants signed informed consent forms. Consent for publication: Not applicable. Competing interests: We declare no competing interests.
Figures
Similar articles
-
Association of HIV Preexposure Prophylaxis Use With HIV Incidence Among Men Who Have Sex With Men in China: A Nonrandomized Controlled Trial.JAMA Netw Open. 2022 Feb 1;5(2):e2148782. doi: 10.1001/jamanetworkopen.2021.48782. JAMA Netw Open. 2022. PMID: 35171258 Free PMC article.
-
Protocol for a multicenter, real-world study of HIV pre-exposure prophylaxis among men who have sex with men in China (CROPrEP).BMC Infect Dis. 2019 Aug 15;19(1):721. doi: 10.1186/s12879-019-4355-y. BMC Infect Dis. 2019. PMID: 31416439 Free PMC article.
-
Using the adherence-efficacy relationship of emtricitabine and tenofovir disoproxil fumarate to calculate background hiv incidence: a secondary analysis of a randomized, controlled trial.J Int AIDS Soc. 2021 May;24(5):e25744. doi: 10.1002/jia2.25744. J Int AIDS Soc. 2021. PMID: 34021709 Free PMC article. Clinical Trial.
-
Preexposure Prophylaxis for the Prevention of HIV: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force.JAMA. 2023 Aug 22;330(8):746-763. doi: 10.1001/jama.2023.9865. JAMA. 2023. PMID: 37606667
-
Safety of oral tenofovir disoproxil fumarate-based HIV pre-exposure prophylaxis use in lactating HIV-uninfected women.Expert Opin Drug Saf. 2017 Jul;16(7):867-871. doi: 10.1080/14740338.2017.1338271. Epub 2017 Jun 8. Expert Opin Drug Saf. 2017. PMID: 28571500 Free PMC article. Review.
References
-
- Hsu YC, Huang DQ, Nguyen MH. Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat Rev Gastroenterol Hepatol. 2023;20:524–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82073620/the Fund of National Natural Science Foundation of China
- 2022JH2/20200074/the program of Department of Science and Technology of Liaoning Province Project for the High-Quality Scientific and Technological Development of China Medical University
- Global Health Governance-02-12/the Public Health Talent Grant by Beijing Municipal Health Commission
- 82473696/Fund of National Natural Science Foundation of China
- 2023-PT320-01/the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

