TDP-43 seeding activity in the olfactory mucosa of patients with amyotrophic lateral sclerosis
- PMID: 40287755
- PMCID: PMC12034174
- DOI: 10.1186/s13024-025-00833-0
TDP-43 seeding activity in the olfactory mucosa of patients with amyotrophic lateral sclerosis
Abstract
Background: In recent years, the seed amplification assay (SAA) has enabled the identification of pathological TDP-43 in the cerebrospinal fluid (CSF) and olfactory mucosa (OM) of patients with genetic forms of frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS). Here, we investigated the seeding activity of TDP-43 in OM samples collected from patients with sporadic ALS.
Methods: OM samples were collected from patients with (a) sporadic motor neuron diseases (MND), including spinal ALS (n = 35), bulbar ALS (n = 18), primary lateral sclerosis (n = 10), and facial onset sensory and motor neuronopathy (n = 2); (b) genetic MND, including carriers of C9orf72exp (n = 6), TARDBP (n = 4), SQSTM1 (n = 3), C9orf72exp + SQSTM1 (n = 1), OPTN (n = 1), GLE1 (n = 1), FUS (n = 1) and SOD1 (n = 4) mutations; (c) other neurodegenerative disorders (OND), including Alzheimer's disease (n = 3), dementia with Lewy bodies (n = 8) and multiple system atrophy (n = 6); and (d) control subjects (n = 22). All samples were subjected to SAA analysis for TDP-43 (TDP-43_SAA). Plasmatic levels of TDP-43 and neurofilament-light chain (NfL) were also assessed in a selected number of patients.
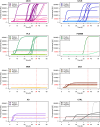
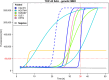
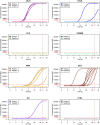
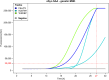
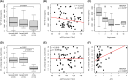
Results: TDP-43_SAA was positive in 29/65 patients with sporadic MND, 9/21 patients with genetic MND, 6/17 OND patients and 3/22 controls. Surprisingly, one presymptomatic individual also tested positive. As expected, OM of genetic non-TDP-43-related MND tested negative. Interestingly, fluorescence values from non-MND samples that tested positive were consistently and significantly lower than those obtained with sporadic and genetic MND. Furthermore, among TDP-43-positive samples, the lag phase observed in MND patients was significantly longer than that in non-MND patients. Plasma TDP-43 levels were significantly higher in sporadic MND patients compared to controls and decreased as the disease progressed. Similarly, plasma NfL levels were higher in both sporadic and genetic MND patients and positively correlated with disease progression rate (ΔFS). No significant correlations were detected between TDP-43_SAA findings and the biological, clinical, or neuropsychological parameters considered.
Conclusions: The OM of a subset of patients with sporadic MND can trigger seeding activity for TDP-43, as previously observed in genetic MND. Thus, TDP-43_SAA analysis of OM can improve the clinical characterization of ALS across different phenotypes and enhance our understanding of these diseases. Finally, plasma TDP-43 could serve as a potential biomarker for monitoring disease progression. However, further research is needed to confirm and expand these findings.
Keywords: Amyotrophic lateral sclerosis; Neurodegeneration; Olfactory mucosa; Peripheral biomarkers; Seed amplification assay; TDP-43.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Comitato Etico Territoriale Lombardia 4 (CET 4) and performed in line with the principles of the Declaration of Helsinki. All subjects were included according to the study protocol and gave written informed consent to the study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Huisman MHB, de Jong SW, van Doormaal PTC, et al. Population based epidemiology of amyotrophic lateral sclerosis using capture-recapture methodology. J Neurol Neurosurg Psychiatry. 2011;82(10):1165–70. 10.1136/jnnp.2011.244939. - PubMed
-
- Gordon PH, Cheng B, Katz IB, Mitsumoto H, Rowland LP. Clinical features that distinguish PLS, upper motor neuron–dominant ALS, and typical ALS. Neurology. 2009;72(22):1948–52. 10.1212/WNL.0b013e3181a8269b. - PubMed
-
- Gordon PH, Cheng B, Katz IB, et al. The natural history of primary lateral sclerosis. Neurology. 2006;66(5):647–53. 10.1212/01.wnl.0000200962.94777.71. - PubMed
-
- Swinnen B, Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nat Rev Neurol. 2014;10(11):661–70. 10.1038/nrneurol.2014.184. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

