Post-marketing quality assessment of some brands of rosuvastatin tablets available in Jos, North-Central Nigeria
- PMID: 40287767
- PMCID: PMC12034138
- DOI: 10.1186/s13065-025-01470-w
Post-marketing quality assessment of some brands of rosuvastatin tablets available in Jos, North-Central Nigeria
Abstract
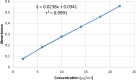
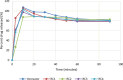
Rosuvastatin is a synthetic statin medication approved for the management of lipid disorders and also for preventing cardiovascular disease in at-risk individuals. Generic rosuvastatin formulations have been developed which are comparatively lower in cost and also assumed to be bio-similar to the innovator brand Crestor®. The present study investigated the chemical and physical attributes together with the in vitro bioequivalence profiles of four generic brands of rosuvastatin calcium tablets marketed in Jos, Nigeria in comparison to the reference brand. The tablet dimensions (thickness and diameter), weight variation, friability, hardness, disintegration time and dissolution profiles were evaluated in accordance to standard procedures. The samples were also assayed using Ultraviolet-Visible spectrophotometry at wavelength of 242.5 nm in methanol. In vitro bioequivalence was evaluated by determining the difference ( ) and similarity ( ) factors. The generic brands all complied with the pharmacopoeial specifications for weight variation, friability and disintegration. In addition, the tablet brands tested all had active drug content ranging from 94.92 to 109.2% and released over 80% of rosuvastatin calcium within the first twenty minutes of the dissolution studies thereby complying with pharmacopoeial requirements for content and dissolution respectively. All brands had similarity factor ( ) values ranging from 50 to 100 and difference factor ( ) values between 0 to 15% at pH 6.6, thus implying that the brands can be used interchangeably with the innovator brand. The chemical and physical tests carried out reveal that the locally marketed brands of rosuvastatin calcium are of good quality and meet the required regulatory standards.
Keywords: Bioequivalence; Dyslipidemia; Nigeria; Quality; Rosuvastatin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not Applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1-45. - PubMed
-
- de Brito Alves JL, Trindade da Costa PC. Probiotic for dyslipidemia prevention and treatment. London: Academic Press; 2022.
LinkOut - more resources
Full Text Sources
Miscellaneous
