Regulation of Genome Architecture in Huntington's Disease
- PMID: 40287840
- PMCID: PMC12060273
- DOI: 10.1021/acs.biochem.5c00029
Regulation of Genome Architecture in Huntington's Disease
Abstract
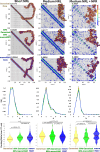
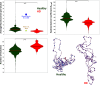
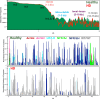
Huntington's disease (HD) is a neurological condition caused by an excessive expansion of CAG repeats in the Huntingtin (HTT) gene. Although experiments have shown an altered epigenetic landscape and chromatin architecture upon HD development, the structural consequences on the HTT gene remain elusive. Structural data are only available for model nucleosome systems and yeast systems with human nucleosomes. Here, we use our experimentally validated nucleosome-resolution mesoscale chromatin model to investigate folding changes of the HTT gene associated with HD. We investigate how the histone fold domain of the variant macroH2A1, a biomarker of HD, affects the genome structure by modeling HD-like systems that contain (i) 100% canonical, (ii) 100% macroH2A1, (iii) 50% canonical and 50% macroH2A1, and (iv) 100% hybrid cores (one canonical H2A and one macroH2A1 per nucleosome). Then, we model the mouse HTT gene in healthy and HD conditions by incorporating the CAG expansion and macroH2A1 cores, reducing the linker histone density and tail acetylation levels, and incorporating genomic contacts. Overall, our results show that the histone fold domain of macroH2A1 affects chromatin compaction in a fiber-dependent manner (i.e., nucleosome distribution dependent) and can thus both enhance or repress HTT gene expression. Our modeling of the HTT gene shows that HTT is less compact in the diseased condition, which could accelerate the production of the mutated protein. By suggesting the structural biophysical consequences of the HTT gene under HD conditions, our findings may help in the development of diagnostic and therapeutic treatments for HD.
Copyright © 2025 The Authors. Published by American Chemical Society. This publication is licensed under CC-BY 4.0.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- MacDonald M. E.; Ambrose C. M.; Duyao M. P.; Myers R. H.; Lin C.; Srinidhi L.; Barnes G.; Taylor S. A.; James M.; Groot N.; MacFarlane H.; Jenkins B.; Anderson M. A.; Wexler N. S.; Gusella J. F. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 1993, 72, 971–983. 10.1016/0092-8674(93)90585-E. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

