Impact of Apolipoprotein A-I Infusions on Cardiovascular Events Post-MI by Neutrophil-Lymphocyte Ratio and LDL-Cholesterol Levels
- PMID: 40288083
- PMCID: PMC12059331
- DOI: 10.1016/j.jacadv.2025.101727
Impact of Apolipoprotein A-I Infusions on Cardiovascular Events Post-MI by Neutrophil-Lymphocyte Ratio and LDL-Cholesterol Levels
Abstract
Background: The AEGIS-II (ApoA-I Event Reducing in Ischemic Syndromes-II; NCT03473223) trial evaluated CSL112, a human plasma-derived apolipoprotein A-I therapy, for reducing cardiovascular events after acute myocardial infarction (AMI). Given CSL112's potential anti-inflammatory properties, we conducted an exploratory post hoc analysis to determine if its efficacy is influenced by baseline neutrophil-lymphocyte ratio (NLR), a marker of systemic inflammation, and low-density lipoprotein cholesterol (LDL-C).
Objectives: The purpose of this study was to investigate the association of baseline NLR and cardiovascular events and explore whether NLR and LDL-C modify CSL112's efficacy in post-AMI patients.
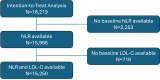
Methods: A total of 18,219 participants with AMI, multivessel coronary artery disease, and additional cardiovascular risk factors were randomized to 4 weekly infusions of 6 g CSL112 or placebo. The primary endpoint was a composite of cardiovascular death, myocardial infarction, or stroke (major adverse cardiovascular events [MACE]). Cox proportional hazards models evaluated risk by dichotomized baseline NLR (>median vs ≤median). Treatment interactions with NLR and LDL-C (≥100 vs <100 mg/dL) were assessed.
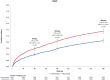
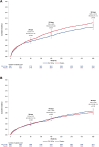
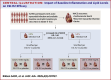
Results: Among 15,966 participants, those with baseline NLR >median (>3.3) had a significantly greater risk of MACE at 90 days (HR: 1.40; 95% CI: 1.21-1.63), persisting at 180 and 365 days. CSL112 reduced MACE at 90 days among participants with elevated NLR and LDL-C ≥100 mg/dL (HR: 0.63; 95% CI: 0.42-0.93), with sustained benefits at 180 and 365 days. Significant interactions were observed between treatment and NLR (Pinteraction = 0.010) and among treatment, NLR, and LDL-C at 180 days (Pinteraction = 0.029).
Conclusions: Baseline elevated NLR predicts MACE in post-AMI patients, and CSL112 showed an associated reduction in MACE in patients with elevated NLR and LDL-C ≥100 mg/dL.
Keywords: CSL112; MACE; apolipoprotein A-I; inflammation; neutrophil-lymphocyte ratio.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding support and author disclosures This study was funded by CSL Behring. Dr Rikken has received speaking honoraria from Daiichi Sankyo. Dr Gibson has received research funding from CSL Behring, Janssen Pharmaceuticals, Johnson & Johnson Corporation, and SCAD Alliance; has provided consulting services for Angel/Avertix Medical Corporation, AstraZeneca, Bayer Corporation, Beren Therapeutics, Boehringer Ingelheim, Boston Clinical Research Institute, Boston Scientific, Bristol Myers Squibb, Cardiovascular Research Foundation, CeleCor Therapeutics, CSL Behring, DCRI, Esperion, EXCITE International (for which no compensation was received), Fortress Biotech, Gilead Sciences, Janssen Pharmaceuticals, MashUp MD, MD Magazine, Microport, MJHealth, Novartis, Novo Nordisk, Pfizer, PHRI, PLxPharma, SCAI, Solstice Health/New Amsterdam Pharma, Somahlution/Marizyme, Vectura, WebMD, and Women as One; holds equity in nference, Dyad Medical, Absolutys, and Fortress Biotech; and has received royalties as a contributor to UpToDate. Dr Bahit has received modest honorarium from MSD, Pfizer, Bristol Myers Squibb, CSL Behring, Janssen, Boehringer Ingelheim, and Anthos Therapeutics. Dr Duffy is an employee of CSL Behring. Dr Chi has received research grant support paid to the Beth Israel Deaconess Medical Center, Harvard Medical School from Bayer, Janssen Scientific Affairs, and CSL Behring. Dr Korjian has received research grant support paid to the Beth Israel Deaconess Medical Center, Harvard Medical School from CSL Behring. Dr White has received grant support to institution from Sanofi, Regeneron Pharmaceuticals, Eli Lilly, Omthera Pharmaceuticals, American Regent, Eisai Inc, DalCor Pharma UK Inc, CSL Behring, NHI, Sanofi-Aventis Australia Pty Ltd, Esperion Therapeutics Inc, and National Institutes of Health; has received fees for serving on Steering Committees of the ODYSSEY trial from Sanofi and Regeneron Pharmaceuticals, the ISCHEMIA and the MINT studies from the National Institutes of Health, the STRENGTH trial from Omthera Pharmaceuticals, the HEART-FID study from American Regent, the DAL-GENE study from DalCor Pharma UK Inc, the AEGIS-II study from CSL Behring, the SCORED trial and the SOLOIST-WHF trial from Sanofi Australia Pty Ltd, and the CLEAR OUTCOMES study from Esperion Therapeutics. Dr Anschuetz has received a salary as an employee of CSL Behring. Dr Kingwell is an employee and shareholder of CSL Ltd. Dr Nicolau has received a scholarship from the National Council of Scientific and Technological Development (CNPq) #303448/2021-0; has received research grants from Amgen, AstraZeneca, Bayer, CSL Behring, Daiichi Sankyo, Dalcor, Esperion, Ionis, Janssen, Novartis, Novo Nordisk, Sanofi, and Vifor; and has received consulting fees from Libbs. Dr Lopes has received grant support from Amgen, Bristol Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi; has been a consultant for AstraZeneca, Bayer, Boehringer, Bristol Myers Squibb, and Novo Nordisk; and has participated in educational activities for Daiichi Sankyo, AstraZeneca, Novo Nordisk, and Pfizer. Dr Lewis has received consulting fees from Janssen R&D and Idorsia. Dr Vinereanu has received research grants from CSL Behring, Bayer, Novartis, Amgen, and Boehringer Ingelheim; and has received consultancy fees from Bayer, Novartis, Amgen, and Boehringer Ingelheim. Dr ten Berg has received institutional research grants from AstraZeneca, Daiichi Sankyo, and ZonMw and personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, CeleCor Therapeutics, and Daiichi Sankyo. Dr Goodman has received research grant support (eg, steering committee or data and safety monitoring committee) and/or speaker/consulting honoraria (eg, advisory boards) from Alnylam, Amgen, Anthos Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, CYTE Ltd, Daiichi Sankyo/American Regent, Eli Lilly, Esperion, Ferring Pharmaceuticals, HLS Therapeutics, Idorsia, JAMP Pharma, Merck, Novartis, Novo Nordisk A/C, Pendopharm/Pharmascience, Pfizer, Regeneron, Roche, Sanofi, Servier, Tolmar Pharmaceuticals, Valeo Pharma; has received salary support/honoraria from the Canadian Heart Failure Society, Canadian Heart Research Centre and MD Primer, Canadian VIGOUR Centre, Cleveland Clinic Coordinating Centre for Clinical Research, Duke Clinical Research Institute, Jewish General Hospital\CIUSSS Centre-Ouest-de-l'Ile-de-Montreal, New York University Clinical Coordinating Centre, PERFUSE Research Institute, Peter Munk Cardiac Centre Clinical Trials and Translation Unit, Ted Rogers Centre for Heart Research, and TIMI Study Group (Brigham Health). Dr Bode has received research support from CSL Behring. Dr Steg has received research grants from Amarin and Sano; has served on clinical trials (steering committee, clinical events committee, data and safety monitoring board [DSMB]) for Amarin, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, CSL Behring, Idorsia, Janssen, Novartis, Novo Nordisk, Pfizer, and Sano; has served as a consultant or speaker for Amarin, Amgen, BMS, and Novo Nordisk; and is Senior Associate Editor at Circulation. Dr Libby has served on a scientific advisory board for Amgen, Kowa Pharmaceuticals, Novo Nordisk, Caristo, CSL Behring, DalCor, Dewpoint, Euclid Bioimaging, Xbiotech, Olatec, Medimmune, PlaqueTec, Polygon Therapeutics, TenSixteen Bio, and Soley Therapeutics; and has been a consultant for Amgen, Baim Institute Beren, Esperion, Genentech, Kancera, Kowa Pharmaceuticals, Novo Nordisk, Novartis, and Sanofi-Regeneron. Dr Bainey has received research support from CSL Behring. Dr van ‘t Hof received a consultancy fee from CeleCor as well as unrestricted grants from Boehringer and Abbott Vascular. Dr Ridker has received institutional research grant support from Kowa, Novartis, Amarin, Pfizer, Esperion, Novo Nordisk, and the NHLBI; has served as a consultant to numerous companies including Novartis, Flame, Agepha, Ardelyx, Arrowhead, AstraZeneca, CSL Behring, Janssen, Civi Biopharm, GlaxoSmithKline, SOCAR, Novo Nordisk, Health Outlook, Montai Health, Eli Lilly, New Amsterdam, Boehringer Ingelheim, RTI, Zomagen, Cytokinetics, Horizon Therapeutics, and Cardio Therapeutics; holds minority shareholder equity positions in Uppton, Bitteroot Bio, and Angiowave; and has received compensation for service on the Peter Munk Advisory Board (University of Toronto), the Leducq Foundation (Paris, FR), and the Baim Institute (Boston, Massachusetts). Dr Mahaffey has received grants from AHA, Apple Inc, Bayer, California Institute for Regenerative Medicine, CSL Behring, Eidos, Ferring, Gilead, Google (Verily), Idorsia, Johnson & Johnson, Luitpold, Novartis, PAC-12, Precordior, Sanifit; has received consulting fees from applied Therapeutics, Bayer, BMS, BridgeBio, CSL Behring, Elsevier, Fosun Pharma, Human, Johnson & Johnson, Moderna, Myokardia, Novartis, Novo Nordisk, Otsuka, Phasebio, Portola, Quidel, and Theravance; has received payment or honoraria from CSL Behring; and has stock or stock options in Human, Medeloop, Precordior, and Regencor. Dr Nicholls has received research support from AstraZeneca, Amgen, Anthera, CSL Behring, Cerenis, Eli Lilly, Esperion, Resverlogix, Novartis, InfraReDx, and Sanofi-Regeneron; and has been a consultant for Amgen, Akcea, AstraZeneca, Boehringer Ingelheim, CSL Behring, Daiichi Sankyo, Eli Lilly, Esperion, Kowa, Merck, Takeda, Pfizer, Sanofi-Regeneron, Novo Nordisk, CSL Sequiris, and Vaxxinity. Dr Mehran has received institutional research payments from Abbott, Affluent Medical, Alleviant Medical, Amgen, AstraZeneca, BAIM, Beth Israel Deaconess Medical Center, Boston Scientific, Bristol Myers Squibb, CardiaWave, CERC, Chiesi, Concept Medical, Daiichi Sankyo, Duke, Faraday, Idorsia, Janssen, MedAlliance, Medscape, Mediasphere, Medtelligence, Medtronic, Novartis, OrbusNeich, Pi-Cardia, Protembis, RM Global Bioaccess Fund Management, and Sanofi; has received personal fees from Affluent Medical, Boehringer Ingelheim, Chiesi USA, Cordis, Daiichi Sankyo, Esperion Science/Innovative Biopharma, Gaffney Events, Educational Trust, Global Clinical Trial Partners, Ltd, IQVIA, Medscape/WebMD Global, Novo Nordisk, PeerView Institute for Medical Education, TERUMO Europe N.V., and Radcliffe; has held equity <1% in Elixir Medical, Stel, ControlRad (spouse); has received an honorarium from AMA; is associate editor of JAMA Cardiology; and is a BOT Member, SC Member CTR Program of ACC. Dr Harrington has research relationships with Baim Institute, CSL, Janssen, NHLBI, PCORI, and DCRI; has consulting relationships with Atropos Health, Bitterroot Bio, BMS, BridgeBio, Element Science, Edwards Lifesciences, Foresite Labs, and Medscape/WebMD; and serves on boards of directors for the American Heart Association, College of the Holy Cross, and Cytokinetics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

References
-
- Steen D.L., Khan I., Andrade K., Koumas A., Giugliano R.P. Event rates and risk factors for recurrent cardiovascular events and mortality in a contemporary post acute coronary syndrome population representing 239 234 patients during 2005 to 2018 in the United States. J Am Heart Assoc. 2022;11(9) - PMC - PubMed
-
- Lawton J.S., Tamis-Holland J.E., Bangalore S., et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79(2):e21–e129. - PubMed
-
- Byrne R.A., Rossello X., Coughlan J.J., et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44(38):3720–3826. - PubMed
-
- Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. - PubMed
-
- Bahit M.C., Gibson C.M. Thrombin as target for prevention of recurrent events after acute coronary syndromes. Thromb Res. 2024;235:116–121. - PubMed
LinkOut - more resources
Full Text Sources

