Dietary Supplements for Endometriosis-Associated Pain: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials
- PMID: 40288359
- PMCID: PMC12158398
- DOI: 10.1159/000545414
Dietary Supplements for Endometriosis-Associated Pain: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials
Abstract
Introduction: In recent years, dietary supplements have emerged as popular "natural" alternatives to conventional pharmacological treatments for various conditions, including endometriosis. The growing popularity of supplements for endometriosis-associated pain, fueled by an expanding and minimally regulated market, underscores the need for robust evidence of efficacy, as a prerequisite for any consideration on effectiveness. This meta-analysis synthesizes evidence from randomized, placebo-controlled trials (RCTs), the gold standard in evidence-based medicine, to assess the efficacy of dietary supplements in endometriosis-associated pain.
Methods: A systematic search of PubMed, Embase, Scopus, and the Cochrane Library was conducted up to November 5, 2024, in adherence to PRISMA 2020 guidelines. Two independent reviewers screened studies using PICOS criteria: reproductive-age women with endometriosis (Population), dietary supplements (Intervention), placebo (Comparator), and pain-related outcomes (Outcomes), assessed in placebo-controlled RCTs adhering to CONSORT standards (Study type). Three pain domains were evaluated: (i) symptom severity (visual analogue scale [VAS] for pelvic pain, dysmenorrhea, dyspareunia), (ii) pain catastrophizing, and (iii) quality of life, as measured by the Short Form-12 Health Survey (SF-12) and the Endometriosis Health Profile-30 (EHP-30). Risk of bias was assessed using the Cochrane RoB2 tool. Random-effects models were used to calculate pooled mean differences (MDs) and 95% confidence intervals (CIs). Statistical heterogeneity was assessed with the I2 statistic, and subgroup analyses explored clinically relevant confounders. Sensitivity analyses excluded studies with conflicts of interest or trustworthiness issues, as defined by the Obstetrics and Gynecology Editors' Integrity Group (OGEIG). Publication bias was evaluated using Egger's test, Begg's test, and the trim-and-fill method. All analyses were conducted using STATA version 18.
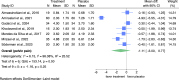
Results: Nine RCTs (n = 545 subjects; 274 in the treatment group and 271 in the placebo group) were included. Only three met the "absolute" OGEIG trustworthiness criteria. No significant differences were observed between supplements and placebo for pelvic pain (pooled MD: -1.1; 95% CI: -3.0 to 0.8; I2 = 96.1%), dysmenorrhea (pooled MD: -2.0; 95% CI: -4.4 to 0.5; I2 = 93.8%), or dyspareunia (pooled MD: -2.0; 95% CI: -4.9 to 0.9; I2 = 96.5%). These findings remained consistent when the analysis was restricted to studies without conflicts of interest, those authored by researchers with no retractions, and those meeting OGEIG trustworthiness criteria. Subgroup analyses reduced heterogeneity and confirmed no significant benefits. Pain catastrophizing and quality-of-life measures showed little to no improvement.
Conclusion: While limited evidence precludes definitive conclusions about specific dietary supplements, available data suggest they lack efficacy for managing endometriosis-associated pain. Given the absence of demonstrated benefits, along with potential harms and costs, dietary supplements should not be recommended at this time for managing endometriosis-related pain.
Keywords: Dietary supplements; Endometriosis; Meta-analysis; Pelvic pain; Systematic review.
© 2025 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
P.Ve. is a member of the Editorial Board of
Figures


Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: An Updated Systematic Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. PMID: 30354042 Free Books & Documents. Review.
-
Two-year efficacy and safety of relugolix combination therapy in women with endometriosis-associated pain: SPIRIT open-label extension study.Hum Reprod. 2024 Mar 1;39(3):526-537. doi: 10.1093/humrep/dead263. Hum Reprod. 2024. PMID: 38243752 Free PMC article. Clinical Trial.
-
Pre- and postsurgical medical therapy for endometriosis surgery.Cochrane Database Syst Rev. 2020 Nov 18;11(11):CD003678. doi: 10.1002/14651858.CD003678.pub3. Cochrane Database Syst Rev. 2020. PMID: 33206374 Free PMC article.
-
The effect of antioxidant supplementation on dysmenorrhea and endometriosis-associated painful symptoms: a systematic review and meta-analysis of randomized clinical trials.Obstet Gynecol Sci. 2024 Mar;67(2):186-198. doi: 10.5468/ogs.23210. Epub 2024 Jan 15. Obstet Gynecol Sci. 2024. PMID: 38221738 Free PMC article.
References
-
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382(13):1244–56. - PubMed
-
- National Institute of Health and Care Excellence . NICE Guideline, No. 73. Endometriosis: diagnosis and management. NICE; [cited 2024 Dec 6]. Available from: https://www.nice.org.uk/guidance/ng73 - PubMed
-
- Collaborative Group on Hormonal Factors in Breast Cancer . Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713–27. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

