Magnetic-Field-Assisted Fe Nanowire Conformable Aerogels Galvanically Displaced to Cu and Pt for Three-Dimensional Electrode Applications
- PMID: 40289324
- PMCID: PMC12067370
- DOI: 10.1021/acsami.5c00693
Magnetic-Field-Assisted Fe Nanowire Conformable Aerogels Galvanically Displaced to Cu and Pt for Three-Dimensional Electrode Applications
Abstract
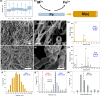
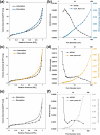
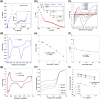
There is an increasing need for free-standing, conformal electrodes for practical energy storage devices. To address this, we demonstrate the magnetic-field-assisted synthesis of interpenetrating Fe nanowire (FeNW) gels without the use of templates or composite scaffold material over a range of magnetic fields. In either a wet gel or a supercritical dried state as an aerogel, the FeNWs may be pressed into thin or conformal films. Varying the applied magnetic field strength with a solenoid during chemical synthesis resulted in increased nanowire length and local orientation of the FeNWs with increasing magnetic field strength, with approximately 80 nm diameters across field strengths of 0-150 mT. Flowing K2PtCl4 or CuSO4·5H2O solutions through the wet iron gels to achieve the near complete galvanic displacement of iron to the more noble [PtCl4]2- and Cu2+ ions resulted in either platinum nanotubes (PtNTs) or copper nanowires (CuNWs) while maintaining a percolating network structure. Similar to the FeNW gels, the PtNT and CuNW gels were able to be supercritical dried and/or pressed into thin or conformal electrode films. CuNW and PtNT films demonstrated good potential as capacitive and oxygen reduction reaction electrodes, respectively. The magnetic-field-assisted synthesis of ferromagnetic iron nanowires offers a simple, rapid, and tunable method that, when combined with galvanic displacement with more noble metal ions, may enable a wide range of metal, alloy, and multimetallic nanowires and nanotubes for energy storage, sensing, and catalytic applications.
Keywords: 3D electrodes; aerogels; copper; fuel cells; iron; nanotubes; nanowires; platinum.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Platinum-coated copper nanowires with high activity for hydrogen oxidation reaction in base.J Am Chem Soc. 2013 Sep 11;135(36):13473-8. doi: 10.1021/ja405598a. Epub 2013 Aug 29. J Am Chem Soc. 2013. PMID: 23952885
-
Robust and Stable Cu Nanowire@Graphene Core-Shell Aerogels for Ultraeffective Electromagnetic Interference Shielding.Small. 2018 Jun;14(23):e1800634. doi: 10.1002/smll.201800634. Epub 2018 May 10. Small. 2018. PMID: 29749012
-
Copper Nanowire-Based Aerogel with Tunable Pore Structure and Its Application as Flexible Pressure Sensor.ACS Appl Mater Interfaces. 2017 Apr 26;9(16):14273-14280. doi: 10.1021/acsami.7b02087. Epub 2017 Apr 17. ACS Appl Mater Interfaces. 2017. PMID: 28398033
-
Advancements in Copper Nanowires: Synthesis, Purification, Assemblies, Surface Modification, and Applications.Small. 2018 Jun;14(26):e1800047. doi: 10.1002/smll.201800047. Epub 2018 Apr 30. Small. 2018. PMID: 29707894 Review.
-
Fabrication and application of macroscopic nanowire aerogels.Nanoscale. 2021 Apr 30;13(16):7430-7446. doi: 10.1039/d0nr09236c. Nanoscale. 2021. PMID: 33928971 Review.
References
-
- Li S.; Dong R.; Li Y.; Lu X.; Qian J.; Wu F.; Wu C.; Bai Y. Advances in Free-Standing Electrodes for Sodium Ion Batteries. Mater. Today 2024, 72, 207–234. 10.1016/j.mattod.2023.11.013. - DOI
-
- Shi T.; Tu Q.; Tian Y.; Xiao Y.; Miara L. J.; Kononova O.; Ceder G. High Active Material Loading in All-Solid-State Battery Electrode via Particle Size Optimization. Adv. Energy Mater. 2020, 10 (1), 190288110.1002/aenm.201902881. - DOI
-
- Sun H.; Zhu J.; Baumann D.; Peng L.; Xu Y.; Shakir I.; Huang Y.; Duan X. Hierarchical 3D Electrodes for Electrochemical Energy Storage. Nat. Rev. Mater. 2019, 4 (1), 45–60. 10.1038/s41578-018-0069-9. - DOI
LinkOut - more resources
Full Text Sources

