Longitudinal investigation of gait and Alzheimer's disease in adults with Down syndrome
- PMID: 40289844
- PMCID: PMC12035545
- DOI: 10.1002/alz.70211
Longitudinal investigation of gait and Alzheimer's disease in adults with Down syndrome
Abstract
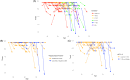
Introduction: Gait abnormalities are associated with Alzheimer's disease (AD) in the general population, but it is unclear if the same is true for individuals with Down syndrome (DS). This study examined gait across 32 months in relation to neuroimaging biomarkers (amyloid beta [Aβ], neurofibrillary tangles [NFTs], and hippocampal volume), cognitive decline, and clinical AD status in adults with DS.
Methods: Participants were 218 adults with DS who underwent Aβ and NFT positron emission tomography (PET) and magnetic resonance imaging (MRI) scans, cognitive testing, and gait assessments at baseline and 32 months. Residual change regression models were conducted.
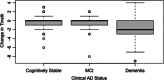
Results: Higher baseline Aβ PET and NFT PET and lower MRI hippocampal volume were associated with gait declines across 32 months. Cognitive declines were associated with gait declines. Participants with clinical dementia at 32 months had greater gait decline than those who were cognitively stable.
Discussion: Gait impairments are a key feature of DS-associated AD (DSAD). Gait assessments could offer a quick, cost-effective, non-invasive screen for DSAD.
Highlights: Those with clinical status of dementia had lower gait performance than those who were cognitively stable. Higher baseline amyloid beta and neurofibrillary tangle volume was associated with more gait impairments. Lower baseline hippocampal volume was associated with more gait impairments. Greater decline in gait performance was associated with cognitive decline. Greater decline in gait performance was associated with more dementia symptoms.
Keywords: amyloid beta; cognitive decline; dementia; gross motor skills; hippocampal volume; neurofibrillary tangles of tau; trisomy 21.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
M.M. received royalties from the University of Rochester and consulting fees from NovoGlia, Inc. and Ireno Health, PBC. S.Z. received royalties from Pavilion Publishing for CAMDEX‐DS‐II, paid to the Horizon‐21 Research Consortium. S.H. received consulting fees from Ionis Pharmaceuticals. M.Z. received consulting fees from LuMind IDSC. E.H. received consulting fees from Cyclo Therapeutics, Alzheon, and Elsevier.
Figures



References
-
- Lejeune J. Etude des chromosomes somatiques de neuf enfants mongoliens. C R Hebd Seances Acad Sci. 1959;248:1713‐1727. - PubMed
-
- Centers for Disease Control and Prevention . Living with Down Syndrome. CDC; 2024. https://www.cdc.gov/birth‐defects/living‐with‐down‐syndrome/?CDC_AAref_V...
MeSH terms
Substances
Grants and funding
- UL1 TR001857/TR/NCATS NIH HHS/United States
- U54 HD087011/HD/NICHD NIH HHS/United States
- U24 AG21886/National Centralized Repository for Alzheimer Disease and Related Dementias
- P30 AG062421/AG/NIA NIH HHS/United States
- P30 AG062715/AG/NIA NIH HHS/United States
- UL1 TR001414/TR/NCATS NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- U01 AG051412/AG/NIA NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- DS-Connect
- UL1 TR002345/TR/NCATS NIH HHS/United States
- U19 AG068054/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P50 HD105353/HD/NICHD NIH HHS/United States
- U54 HD090256/HD/NICHD NIH HHS/United States
- P30 AG066519/AG/NIA NIH HHS/United States
- UL1 TR002373/TR/NCATS NIH HHS/United States
- RO1 AG070028/National Institute on Aging and the National Institute for Child Health and Human Development
- UL1 TR001873/TR/NCATS NIH HHS/United States
- P50 AG16537/Alzheimer's Disease Research Centers Program
- U01 AG051406/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

