Effects of Supplementation with Microalgae Extract from Tetradesmus obliquus Strain Mi175.B1.a on Gastrointestinal Symptoms and Mental Health in Healthy Adults: A Pilot Randomized, Double-Blind, Placebo-Controlled, Parallel-Arm Trial
- PMID: 40289936
- PMCID: PMC11944429
- DOI: 10.3390/nu17060960
Effects of Supplementation with Microalgae Extract from Tetradesmus obliquus Strain Mi175.B1.a on Gastrointestinal Symptoms and Mental Health in Healthy Adults: A Pilot Randomized, Double-Blind, Placebo-Controlled, Parallel-Arm Trial
Abstract
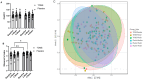
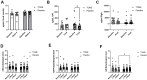
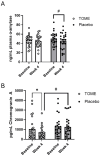
Microalgae, a marine-derived natural ingredient, has emerged as a rich source of bioactive compounds with the potential to modulate gut-brain axis activities. The objective of this study was to investigate whether supplementation with a microalgae extract from Tetradesmus obliquus strain Mi175.B1.a (TOME) influences gut health and reduces stress and anxiety in healthy adults experiencing mild to moderate gastrointestinal (GI) distress. Methods: Fifty-six healthy adults (age: 31.9 ± 7.7 years; body weight: 71.8 ± 12.6 kg; BMI: 24.6 ± 2.8 kg/m2) were enrolled in a randomized, double-blind, placebo-controlled, parallel-arm clinical trial. Participants were randomly allocated to receive capsules containing either 250 mg/day of TOME or a placebo for four weeks. Primary outcomes included the assessment of GI symptoms using the Gastrointestinal Symptom Rating Scale (GSRS) and Bristol Stool Scale (BSS). Secondary outcomes focused on subjective evaluation of mood, stress, and anxiety, as well as blood pressure responses to sympathetic nervous system activation induced by the cold pressor test (CPT). In addition, stool, plasma, and saliva samples were collected to assess biomarkers associated with stress, sympathetic activation, intestinal permeability, and GI health. 16S rRNA sequencing was performed to analyze changes in gut microbial populations. Results: Daily supplementation for four weeks with TOME was safe and well tolerated in the study population. In addition, TOME significantly reduced GSRS global scores (p = 0.02), as well as constipation (p = 0.05) and indigestion (p = 0.03) subcomponent scores compared to Placebo. There was also a significant increase in Shannon's index before FDR correction (p = 0.05; FDR = 0.12) and stool butyrate level was significantly lower in the TOME group than in Placebo after 4 weeks of supplementation (p = 0.039). Both groups showed a significant reduction in perceived stress scores, but the TOME intervention group also had reduced Negative Affect scores (p < 0.001). In addition, plasma chromogranin A, a stress biomarker, was significantly reduced after TOME intervention (p = 0.03). There were no negative effects on blood lipids or other parameters related to sympathetic activation or cardiovascular health. Conclusions: Overall, these results suggest that 4-week supplementation with T. obliquus strain Mi175.B1.a improves GI symptoms, potentially through effects on the gut microbiota, and may promote positive effects on mental health. Additional research should follow up on mental health outcomes in populations with increased stress and anxiety and investigate mechanisms underlying improvements in GI health. This trial was registered at clinicaltrials.gov as NCT06425094.
Keywords: Tetradesmus obliquus; anxiety; blood pressure; gastrointestinal symptoms; gut microbiota; intestinal health; mental health; microalgae; microbiome; phytochemicals; stress; sympathetic nervous system.
Conflict of interest statement
J.M. and R.P. are employees of Microphyt. They provided input on study design and primary outcome measures. J.M. also participated in data analysis and interpretation and manuscript preparation. No other authors have personal or financial conflicts of interest with the study sponsor or product evaluated in this study.
Figures




References
-
- Sperber A.D., Bangdiwala S.I., Drossman D.A., Ghoshal U.C., Simren M., Tack J., Whitehead W.E., Dumitrascu D.L., Fang X., Fukudo S., et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021;160:99–114.e3. doi: 10.1053/j.gastro.2020.04.014. - DOI - PubMed
-
- Fekete M., Lehoczki A., Tarantini S., Fazekas-Pongor V., Csípő T., Csizmadia Z., Varga J.T. Improving Cognitive Function with Nutritional Supplements in Aging: A Comprehensive Narrative Review of Clinical Studies Investigating the Effects of Vitamins, Minerals, Antioxidants, and Other Dietary Supplements. Nutrients. 2023;15:5116. doi: 10.3390/nu15245116. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

