Dietary Polyunsaturated Fatty Acid Deficiency Impairs Renal Lipid Metabolism and Adaptive Response to Proteinuria in Murine Renal Tubules
- PMID: 40289946
- PMCID: PMC11944481
- DOI: 10.3390/nu17060961
Dietary Polyunsaturated Fatty Acid Deficiency Impairs Renal Lipid Metabolism and Adaptive Response to Proteinuria in Murine Renal Tubules
Abstract
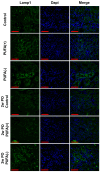
Background/Objectives: Kidneys are fatty acid (FA)-consuming organs that use adenosine triphosphate (ATP) for tubular functions, including endocytosis for protein reabsorption to prevent urinary protein loss. Peroxisome proliferator-activated receptor α (PPARα) is a master regulator of FA metabolism and energy production, with high renal expression. Although polyunsaturated fatty acids (PUFAs) are essential nutrients that are natural PPARα ligands, their role in tubular protein reabsorption remains unclear. As clinical PUFA deficiency occurs in humans under various conditions, we used a mouse model that mimics these conditions. Methods: We administered a 2-week intraperitoneal protein-overload (PO) treatment to mice that had been continuously fed a PUFA-deficient diet. We compared the phenotypic changes with those in mice fed a standard diet and those in mice fed a PUFA-deficient diet with PUFA supplementation. Results: In the absence of PO, the PUFA-deficient diet induced increased lysosomal autophagy activation; however, other phenotypic differences were not detected among the diet groups. In the PO experimental condition, the PUFA-deficient diet increased daily urinary protein excretion and tubular lysosomes; suppressed adaptive endocytosis activation, which was probably enhanced by continuous autophagy activation; and worsened FA metabolism and PPARα-mediated responses to PO, which disrupted renal energy homeostasis. However, these changes were attenuated by PUFA supplementation at the physiological intake level. Conclusions: PUFAs are essential nutrients for the tubular adaptive reabsorption response against urinary protein loss. Therefore, active PUFA intake may be important for patients with kidney disease-associated proteinuria, especially those with various PUFA deficiency-inducing conditions.
Keywords: PPARα; fatty acid metabolism; kidney function; lysosome; tubular protein reabsorption.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









Similar articles
-
Decreased Fatty Acid β-Oxidation Is the Main Cause of Fatty Liver Induced by Polyunsaturated Fatty Acid Deficiency in Mice.Tohoku J Exp Med. 2017 Jul;242(3):229-239. doi: 10.1620/tjem.242.229. Tohoku J Exp Med. 2017. PMID: 28724855
-
Polyunsaturated fatty acid deficiency affects sulfatides and other sulfated glycans in lysosomes through autophagy-mediated degradation.FASEB J. 2020 Jul;34(7):9594-9614. doi: 10.1096/fj.202000030RR. Epub 2020 Jun 5. FASEB J. 2020. PMID: 32501606
-
Activation of peroxisome proliferator-activated receptor alpha by dietary fish oil attenuates steatosis, but does not prevent experimental steatohepatitis because of hepatic lipoperoxide accumulation.J Gastroenterol Hepatol. 2008 Feb;23(2):267-75. doi: 10.1111/j.1440-1746.2007.05157.x. Epub 2007 Sep 12. J Gastroenterol Hepatol. 2008. PMID: 17868330
-
Mechanisms of regulation of gene expression by fatty acids.Lipids. 2004 Nov;39(11):1077-83. doi: 10.1007/s11745-004-1333-0. Lipids. 2004. PMID: 15726822 Review.
-
Fatty acid regulation of hepatic lipid metabolism.Curr Opin Clin Nutr Metab Care. 2011 Mar;14(2):115-20. doi: 10.1097/MCO.0b013e328342991c. Curr Opin Clin Nutr Metab Care. 2011. PMID: 21178610 Free PMC article. Review.
References
-
- Xie S.Y., Liu S.Q., Zhang T., Shi W.K., Xing Y., Fang W.X., Zhang M., Chen M.Y., Xu S.C., Fan M.Q., et al. USP28 serves as a key suppressor of mitochondrial morphofunctional defects and cardiac dysfunction in the diabetic heart. Circulation. 2024;149:684–706. doi: 10.1161/CIRCULATIONAHA.123.065603. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

