Bifidogenic Effect of 2'-Fucosyllactose (2'-FL) on the Gut Microbiome of Healthy Formula-Fed Infants: A Randomized Clinical Trial
- PMID: 40290019
- PMCID: PMC11944528
- DOI: 10.3390/nu17060973
Bifidogenic Effect of 2'-Fucosyllactose (2'-FL) on the Gut Microbiome of Healthy Formula-Fed Infants: A Randomized Clinical Trial
Abstract
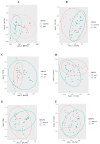
Breast milk is rich in bioactive components, especially human milk oligosaccharides (HMOs), which are crucial for establishing gut microbiota. The 2'-FL (2-Fucosyllactose), one of the most abundant oligosaccharides in breast milk, functions as a selective prebiotic. Objective: To examine the effect of adding 2'-FL (2-Fucosyllactose) to an infant formula containing prebiotic galacto-oligosaccharides (GOSs) and fructo-oligosaccharides (FOSs) on the gut microbiome of healthy formula-fed infants. Methods: This study enrolled infants from three groups: an HMO experimental group (n = 29), a GOS/FOS control group (n = 30), and an exclusively breastfed (breast milk [BM]) reference group (n = 28). Fecal samples from the three groups in the first and fourth months of life were analyzed. The V3 and V4 regions of the 16S rRNA gene were amplified and sequenced on the Illumina MiSeq. ANOVA, Kruskal-Wallis, richness indices (Chao1, Shannon), UniFrac distances, and the Adonis tests were used to perform statistical analyses on the relative abundance of phyla and genera, as well as the alpha and beta-diversity of the gut microbiota. Results: After intervention, Actinobacteriota emerged as the predominant phylum in both the HMO (60.4%) and BM (46.6%) groups. Bifidobacterium and Escherichia-Shigella were identified as the two most abundant bacterial genera in both groups. Nevertheless, the statistical analysis showed that the relative abundance of Bifidobacterium in the HMO formula-fed group after intervention was similar to that in the BM group (p > 0.05). Infants in the HMO and GOS/FOS groups showed higher relative abundance of [Ruminococcus]_gnavus_group bacteria compared to those in the BM group. Groups fed with infant formula demonstrated higher alpha-diversity of gut microbiota compared to breastfed infants (p < 0.05), at the time of admission as well as after the intervention. Beta-diversity was significantly different among the three groups, according to type of feeding. Infants fed a 2'-FL-supplemented infant formula exhibited growth comparable to that of breastfed infants throughout the intervention period, demonstrating that the formula was both safe and well tolerated. Conclusions: Adding 2'-FL to an infant formula containing 4 g/L of GOS + FOS resulted in a stronger bifidogenic effect compared to the formula without 2'-FL.
Keywords: 2-fucosyllactose; breast milk; fructo-oligosaccharides; galacto-oligosaccharides; gut microbiome; human milk oligosaccharides; infant formula.
Conflict of interest statement
T.L. and K.M.T. are the former employees of Nestlé Nutrition, Brazil. All other authors report no conflict of interests concerning this research.
Figures





References
-
- World Health Organization, United Nations Children’s Fund . Global Strategy for Infant and Young Child Feeding. World Health Organization; Geneva, Switzerland: 2003.
-
- BRASIL. Ministério da Saúde. Secretaria de Atenção Primaria à Saúde. Departamento de Promoção da Saúde . Guia Alimentar Para Crianças Brasileiras Menores De 2 Anos. Secretaria de Atenção Primaria à Saúde; Departamento de Promoção da Saúde; Ministério da Saúde; Brasília, Brazil: 2019.
-
- Sociedade Brasileira de Pediatria (SBP) Weffort V.R.S. Manual De Alimentação: Orientações Para Alimentação Do Lactente Ao Adolescente, Na Escola, Na Gestante, Na Prevenção De Doenças E Segurança Alimentar. SBP; São Paulo, Brazil: 2018. Sociedade Brasileira de Pediatria, Departamento Científico de Nutrologia.
-
- ILSI-Brasil . Dinâmica da Composição Do Leite Humano E Suas Implicações Clínicas. ILSI Brasil (International Life Sciences Institute do Brasil); São Paulo, Brazil: 2018.
-
- Weffort V.R.S., Lamounier J.A. Nutrição Em Pediatria: Da Neonatologia À Adolescência. 3rd ed. Manole; São Paulo, Brazil: 2024. pp. 156–185.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

