Efficacy of naloxone in reducing hypoxemia and duration of immobility following focal to bilateral tonic-clonic seizures
- PMID: 40290094
- PMCID: PMC12163546
- DOI: 10.1002/epi4.70046
Efficacy of naloxone in reducing hypoxemia and duration of immobility following focal to bilateral tonic-clonic seizures
Abstract
Objective: Evaluating the efficacy of an opioid antagonist, naloxone (NLX), to reduce the severity of post-ictal hypoxemia and immobility after focal to bilateral tonic-clonic seizures (FBTCS).
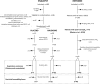
Methods: ENALEPSY is a double-blind placebo (PCB)-controlled trial conducted in patients with focal epilepsy undergoing long-term video-EEG monitoring (LTM). Patients with a FBTCS during LTM were randomized 1:1 to receive intravenous NLX or PCB within the 2 min following the end of FBTCS. After database lock, a discrepancy between the allocated arm and the received treatment was detected, resulting in a 4:1 NLX:PCB ratio. To further explore the efficacy of NLX, we used historical control (HC) data collected in patients included in the REPO2MSE study whose characteristics matched those of patients randomized in ENALEPSY. The efficacy of NLX was then assessed versus PCB and versus HC. The primary endpoint was the delay between the end of the seizure and recovery of SpO2 ≥ 90%. Secondary efficacy outcomes included desaturation nadir and duration of the post-ictal immobility.
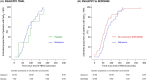
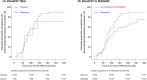
Results: 33 patients contributed to the NLX group, 7 to the PCB group, and 43 to the HC group. The proportion of FBTCS type 1 or 3 was 84% in NLX, 100% in PCB, and 84% in HC. NLX did not improve the delay of recovery of SpO2 ≥ 90% or the desaturation nadir. By contrast, the duration of the post-ictal immobility differed across groups. The time to mobility recovery within the first 5 min post-ictal was very similar in the PCB (200.3 ± 215.8 s) and HC (194.4 ± 192.0 s) groups, and significantly shorter in the NLX group (128.9 ± 151.1 s) when compared to HC (Hazard Ratio, 1.84; 95% CI, 1.11-3.05; p = 0.021).
Significance: NLX did not prevent post-ictal respiratory dysfunction but might reduce the duration of post-ictal immobility. Confirmation of this effect and its impact on SUDEP risk will require additional studies.
Plain language summary: Release of endogenous opioids might participate in the severity of post-ictal hypoxemia and immobility after focal to bilateral tonic-clonic seizures (FBTCS). We conducted a multicenter double-blind randomized placebo-controlled trial evaluating the efficacy of an opioid antagonist, naloxone (NLX), administered within 2 min following the end of FBTCS. The efficacy of NLX was further explored with a comparison with historical control. NLX did not improve the delay of recovery or the severity of post-ictal hypoxemia. Post-ictal immobility was significantly shorter in the NLX group when compared to historical control. The impact of these results on SUDEP prevention will require additional studies.
Keywords: SUDEP; epilepsy; generalized convulsive seizures; naloxone; post‐ictal EEG hypoxemia; post‐ictal immobility.
© 2025 The Author(s). Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
No author has a conflict of interest related to this study. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures
References
-
- Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. 2016;15:1075–1088. - PubMed
-
- Ryvlin P, Cucherat M, Rheims S. Risk of sudden unexpected death in epilepsy in patients given adjunctive antiepileptic treatment for refractory seizures: a meta‐analysis of placebo‐controlled randomised trials. Lancet Neurol. 2011;10:961–968. - PubMed
-
- Thijs RD, Ryvlin P, Surges R. Autonomic manifestations of epilepsy: emerging pathways to sudden death. Nat Rev Neurol. 2021;17:774–788. - PubMed
-
- Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12:966–977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical