Fermented beetroot modulates gut microbial carbohydrate metabolism in prediabetes and prevents high-fat diet induced hyperglycemia in a prediabetic model
- PMID: 40290372
- PMCID: PMC12022487
- DOI: 10.1016/j.crfs.2025.101052
Fermented beetroot modulates gut microbial carbohydrate metabolism in prediabetes and prevents high-fat diet induced hyperglycemia in a prediabetic model
Abstract
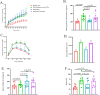
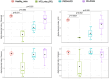
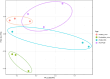
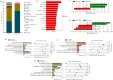
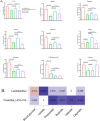
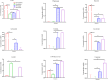
The global increase in prevalence of (pre-)diabetes demands immediate intervention strategies. In our earlier work, we demonstrated in vitro antidiabetic potential of a fermented beetroot product (PN39). Here, we examined the impact of PN39 on glucose tolerance and gut microbiota in C57BL/6J male mice and on prediabetic (PD) subjects' stool microbiota. In mice, high-fat diet (HFD) consumption for 9 weeks resulted in hyperglycemia and impaired glucose tolerance (GT) while concomitant consumption of PN39 and HFD (PN39+HFD) prevented GT impairment. Meanwhile, feeding the mice with HFD for 5 weeks to induce PD and later administering them with PN39 for 4 weeks (PD + PN39) neither improved fasting blood glucose nor GT. Relative to control groups, the gut microbiota of both PD mice and humans were characterized by decreased Clostridia UCG-014 and Lactobacilli as well as significantly altered gut microbial carbohydrate metabolism. Feeding PN39 together with HFD preserved Clostridia UCG-014 and Lactobacilli, increased short chain fatty acid production relative to mice fed with HFD only. Treating gut microbiota of PD subjects with PN39 however increased Clostridia UCG-014 and Lactobacilli populations and increased short chain fatty acids concentrations in the stools. In both mice and humans, PN39 treatment rectified the altered microbial carbohydrate metabolism observed in their PD counterparts. This suggests that the gut microbial modulatory effects of PN39 coupled with its capacity to regulate gut microbial glucose metabolism, likely played a role in preventing PD in mice receiving PN39+HFD. Taken together, our results indicate that PN39 could act as a potent antidiabetic functional food for preventing diabetes and its associated dysbiosis.
Keywords: Dysbiosis; Functional food; Gut microbial metabolism; Gut microbiota; Hyperglycemia.
© 2025 The Authors. Published by Elsevier B.V.
Conflict of interest statement
All authors declare no financial or non-financial competing interests.
Figures


Similar articles
-
A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference.Nutrients. 2020 Oct 20;12(10):3197. doi: 10.3390/nu12103197. Nutrients. 2020. PMID: 33092019 Free PMC article.
-
Amoxicillin modulates gut microbiota to improve short-term high-fat diet induced pathophysiology in mice.Gut Pathog. 2022 Oct 13;14(1):40. doi: 10.1186/s13099-022-00513-0. Gut Pathog. 2022. PMID: 36229889 Free PMC article.
-
Lead exposure aggravates glucose metabolism disorders through gut microbiota dysbiosis and intestinal barrier damage in high-fat diet-fed mice.J Sci Food Agric. 2024 Mar 30;104(5):3057-3068. doi: 10.1002/jsfa.13197. Epub 2023 Dec 17. J Sci Food Agric. 2024. PMID: 38057285
-
Protein quality and the protein to carbohydrate ratio within a high fat diet influences energy balance and the gut microbiota in C57BL/6J mice.PLoS One. 2014 Feb 10;9(2):e88904. doi: 10.1371/journal.pone.0088904. eCollection 2014. PLoS One. 2014. PMID: 24520424 Free PMC article.
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
References
-
- Beresford-Jones B.S., Forster S.C., Stares M.D., Notley G., Viciani E., Browne H.P., Boehmler D.J., Soderholm A.T., Kumar N., Vervier K. The mouse gastrointestinal bacteria catalogue enables translation between the mouse and human gut microbiotas via functional mapping. Cell Host Microbe. 2022;30(1):124–138e128. doi: 10.1016/j.chom.2021.12.003. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

