A real-world pharmacovigilance study of adverse drug reactions associated with lecanemab and aducanumab based on WHO-VigiAccess and FAERS databases
- PMID: 40290431
- PMCID: PMC12023478
- DOI: 10.3389/fphar.2025.1561020
A real-world pharmacovigilance study of adverse drug reactions associated with lecanemab and aducanumab based on WHO-VigiAccess and FAERS databases
Abstract
Background: Lecanemab and Aducanumab are two novel anti-amyloid beta (Aβ) therapies for Alzheimer's disease (AD) that have shown promise in slowing cognitive decline. However, their safety profiles remain unclear due to limited real-world evidence. This study aims to analyze and compare adverse drug reactions (ADRs) of these drugs using data from the WHO-VigiAccess and FAERS databases.
Methods: A retrospective analysis was conducted using ADR data from the VigiAccess and FAERS databases, focusing on System Organ Class (SOC) and Preferred Term (PT) classifications. Descriptive statistics and reporting odds ratio (ROR) analysis were employed to evaluate and compare ADR profiles.
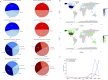
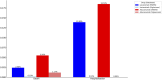
Results: Lecanemab and Aducanumab exhibited distinct ADRs. Results from both the VigiAccess and FAERS databases indicated that the most SOC associated with both drugs was nervous system disorders (34.7% in VigiAccess, 36.8% in FAERS). Further multivariable logistic regression analysis revealed that Aducanumab was associated with a higher risk of nervous system disorders (OR = 4.72, 95% CI: 3.53-6.39, P < 0.001). Among the reported AEs, headache was the most frequently reported for Lecanemab (9.4% in VigiAccess, 8.96% in FAERS), while Aducanumab was primarily associated with amyloid-related imaging abnormalities (ARIA) (19.1% in VigiAccess, 23.58% in FAERS). In the blood and lymphatic systems, Anemia was observed in both drugs. However, thrombocyto-penia was more prevalent in Lecanemab, while platelet dysfunction and myelosuppression were more frequently observed in Aducanumab. Additionally, hospitalization and mortality rates were higher for Aducanumab compared to Lecanemab.
Conclusion: This study compared the ADRs of Lecanemab and Aducanumab, revealing that ARIA was the most common AE for both drugs. However, Lecanemab showed a lower risk of ARIA, cerebral hemorrhage, and severe events. These findings emphasize the need for further clinical research to clarify the long-term safety and efficacy of both drugs.
Keywords: FAERS; VigiAccess; aducanumab; alzheimer’s disease (AD); lecanemab.
Copyright © 2025 Hu, Zhao, Mao, He, Zhang, Ye and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
A real-world disproportionality analysis of FDA adverse event reporting system (FAERS) events for lecanemab.Front Pharmacol. 2025 Apr 2;16:1559447. doi: 10.3389/fphar.2025.1559447. eCollection 2025. Front Pharmacol. 2025. PMID: 40242445 Free PMC article.
-
Comparison of safety of lecanemab and aducanumab: a real-world disproportionality analysis using the FDA adverse event reporting system.Front Pharmacol. 2025 May 30;16:1593989. doi: 10.3389/fphar.2025.1593989. eCollection 2025. Front Pharmacol. 2025. PMID: 40520157 Free PMC article.
-
Real-world study of adverse events associated with gepant use in migraine treatment based on the VigiAccess and U.S. Food and Drug Administration's adverse event reporting system databases.Front Pharmacol. 2024 Jul 31;15:1431562. doi: 10.3389/fphar.2024.1431562. eCollection 2024. Front Pharmacol. 2024. PMID: 39144633 Free PMC article.
-
Comparative efficacy, tolerability, and acceptability of aducanumab, lecanemab, and donanemab with repetitive transcranial magnetic stimulation on cognitive function in mild cognitive impairment and Alzheimer's disease: A systematic review and network meta-analysis.J Psychopharmacol. 2025 May 19:2698811251340901. doi: 10.1177/02698811251340901. Online ahead of print. J Psychopharmacol. 2025. PMID: 40386876 Review.
-
Impact of Anti-amyloid-β Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer's Disease: A Focus on Aducanumab and Lecanemab.Front Aging Neurosci. 2022 Apr 12;14:870517. doi: 10.3389/fnagi.2022.870517. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35493943 Free PMC article. Review.
Cited by
-
Real-world safety profile of mitomycin: signal detection and time-to-onset analysis from FDA adverse event reporting system and VigiAccess databases.Int J Clin Pharm. 2025 Aug 28. doi: 10.1007/s11096-025-01994-0. Online ahead of print. Int J Clin Pharm. 2025. PMID: 40875084
References
-
- Abdelazim K., Allam A. A., Afifi B., Abdulazeem H., Elbehiry A. I. (2024). The efficacy and safety of lecanemab 10 mg/kg biweekly compared to a placebo in patients with alzheimer’s disease: a systematic review and meta-analysis of randomized controlled trials. Neurol. Sci. 45, 3583–3597. 10.1007/s10072-024-07477-w - DOI - PMC - PubMed
-
- Alzheimer’s Association (2024). Aducanumab discontinued as alzheimer’s treatment | alz.org. Available online at: https://www.alz.org/alzheimers-dementia/treatments/aducanumab.
LinkOut - more resources
Full Text Sources
Research Materials

