The molecular determinants regulating redox signaling in diabetic endothelial cells
- PMID: 40290438
- PMCID: PMC12023289
- DOI: 10.3389/fphar.2025.1563047
The molecular determinants regulating redox signaling in diabetic endothelial cells
Abstract
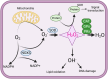
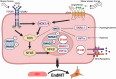
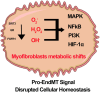
Oxidation and reduction are vital for keeping life through several prime mechanisms, including respiration, metabolism, and other energy supplies. Mitochondria are considered the cell's powerhouse and use nutrients to produce redox potential and generate ATP and H2O through the process of oxidative phosphorylation by operating electron transfer and proton pumping. Simultaneously, mitochondria also produce oxygen free radicals, called superoxide (O2 -), non-enzymatically, which interacts with other moieties and generate reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), peroxynitrite (ONOO-), and hydroxyl radical (OH-). These reactive oxygen species modify nucleic acids, proteins, and carbohydrates and ultimately cause damage to organs. The nutrient-sensing kinases, such as AMPK and mTOR, function as a key regulator of cellular ROS levels, as loss of AMPK or aberrant activation of mTOR signaling causes ROS production and compromises the cell's oxidant status, resulting in various cellular injuries. The increased ROS not only directly damages DNA, proteins, and lipids but also alters cellular signaling pathways, such as the activation of MAPK or PI3K, the accumulation of HIF-1α in the nucleus, and NFkB-mediated transcription of pro-inflammatory cytokines. These factors cause mesenchymal activation in renal endothelial cells. Here, we discuss the biology of redox signaling that underlies the pathophysiology of diabetic renal endothelial cells.
Keywords: AMPK; EndMT; diabetes; endothelial cells; endothelial dysfunctions; mTORC1.
Copyright © 2025 Srivastava, Kopasz-Gemmen, Thurman, Rajendran, Selvam, Kumar, Srivastava, Suresh, Kumari, Goodwin and Inoki.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology.Am J Physiol Regul Integr Comp Physiol. 2004 Nov;287(5):R1014-30. doi: 10.1152/ajpregu.00124.2004. Am J Physiol Regul Integr Comp Physiol. 2004. PMID: 15475499
-
Chemical and Cellular Formation of Reactive Oxygen Species from Secondary Organic Aerosols in Epithelial Lining Fluid.Res Rep Health Eff Inst. 2023 Dec;2023(215):1-56. Res Rep Health Eff Inst. 2023. PMID: 38420854 Free PMC article.
-
Detection of mitochondria-generated reactive oxygen species in cells using multiple probes and methods: Potentials, pitfalls, and the future.J Biol Chem. 2018 Jun 29;293(26):10363-10380. doi: 10.1074/jbc.RA118.003044. Epub 2018 May 8. J Biol Chem. 2018. PMID: 29739855 Free PMC article.
-
Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer.Am J Physiol Heart Circ Physiol. 2008 Feb;294(2):H570-8. doi: 10.1152/ajpheart.01324.2007. Epub 2007 Dec 14. Am J Physiol Heart Circ Physiol. 2008. PMID: 18083891 Review.
-
Oxidants in Physiological Processes.Handb Exp Pharmacol. 2021;264:27-47. doi: 10.1007/164_2020_380. Handb Exp Pharmacol. 2021. PMID: 32767144 Review.
Cited by
-
Exercise orchestrates systemic metabolic and neuroimmune homeostasis via the brain-muscle-liver axis to slow down aging and neurodegeneration: a narrative review.Eur J Med Res. 2025 Jun 12;30(1):475. doi: 10.1186/s40001-025-02751-9. Eur J Med Res. 2025. PMID: 40506775 Free PMC article. Review.
References
-
- Adeshara K. A., Bangar N., Diwan A. G., Tupe R. S. (2022). Plasma glycation adducts and various RAGE isoforms are intricately associated with oxidative stress and inflammatory markers in type 2 diabetes patients with vascular complications. Diabetes Metab. Syndr. 16 (3), 102441. 10.1016/j.dsx.2022.102441 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

