Comparative study of chitosan-based liquid dressing and recombinant human epidermal growth factor on acute limb skin wound healing: A randomized controlled trial
- PMID: 40290458
- PMCID: PMC12033393
- DOI: 10.1016/j.jpra.2025.03.018
Comparative study of chitosan-based liquid dressing and recombinant human epidermal growth factor on acute limb skin wound healing: A randomized controlled trial
Abstract
Background: Several traditional dressings may have limitation in treating wounds. A novel chitosan-based dressing designed for improved hemostasis, moisture, and sealing shows promise in wound healing. However, its efficacy and safety are yet to be sufficiently verified in patients.
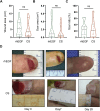
Methods: This randomized controlled trial enrolled 40 patients suffering from acute skin wounds in the limbs from 12/2022 to 12/2023. They were randomly divided into two groups (20 vs. 20) and received regular treatments in the Shenzhen Second People's Hospital. The experimental group was treated with chitosan-based liquid dressing, whereas the control group was treated with traditional dressing with recombinant human epidermal growth factor (rhEGF). The therapeutic effects (scar area and pigment deposition), adverse events, visual analogue scale (VAS), healing time, cost, and the patient and observer scar assessment scale (POSAS) were evaluated on days 0, 7, 14, and 28.
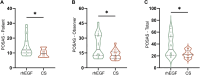
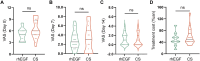
Results: No adverse events were observed throughout the trial. On day 28, effective rate between groups were not statistically significant between the groups (70% vs. 85%, p = 0.256). Other parameters that were not significant included VAS (5.10 ± 1.62 vs. 6.35 ± 2.39, p = 0.06), healing time (8.45 ± 4.26 vs. 8.60 ± 5.44 days, p = 0.923), and cost (49.00 ± 22.48 vs. 57.40 ± 27.59, p = 0.298). However, on day 28, the patient- and observer-reported SAS of the chitosan (CS) group was significantly lower than that of the rhEGF group (12.00 vs. 9.50, z = 2.477, p = 0.013; 18.50 vs. 12.50, z = 2.209, p = 0.026; respectively), and the total POSAS (30.50 vs. 22.00, z = 2.374, p = 0.017).
Conclusion: Compared to rhEGF, the CS-based liquid dressing showed reliable safety and equivalent performance in treating acute limb skin wounds, as revealed by improvements in healing time and rate, pain relief, and costs. Moreover, liquid dressing significantly reduced scar formation, indicating its potential in wound therapy.
Keywords: Chitosan; EGF; Scar; Wound Healing.
© 2025 The Author(s).
Conflict of interest statement
The study was sponsored by Hebei Chuangyue Biotechnology Co., Ltd, who did not participate in this study directly. None of the listed authors were affiliated with this institution. All remaining authors have declared no conflicts of interest.
Figures

Similar articles
-
Chitosan-microencapsulated rhEGF in promoting wound healing.J Wound Care. 2021 Sep 2;30(Sup9a):IXi-IXxi. doi: 10.12968/jowc.2021.30.Sup9a.IX. J Wound Care. 2021. PMID: 34570632
-
[Clinical study of cell sheets containing allogeneic keratinocytes and fibroblasts for the treatment of partial-thickness burn wounds].Zhonghua Shao Shang Za Zhi. 2020 Mar 20;36(3):171-178. doi: 10.3760/cma.j.cn501120-20191113-00426. Zhonghua Shao Shang Za Zhi. 2020. PMID: 32241042 Chinese.
-
Clinical efficacy of wet dressing combined with chitosan wound dressing in the treatment of deep second-degree burn wounds: A prospective, randomised, single-blind, positive control clinical trial.Int Wound J. 2023 Mar;20(3):699-705. doi: 10.1111/iwj.13911. Epub 2022 Aug 3. Int Wound J. 2023. PMID: 35922093 Free PMC article. Clinical Trial.
-
Negative pressure wound therapy for surgical wounds healing by primary closure.Cochrane Database Syst Rev. 2020 May 1;5(5):CD009261. doi: 10.1002/14651858.CD009261.pub5. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Jun 15;6:CD009261. doi: 10.1002/14651858.CD009261.pub6. PMID: 32356396 Free PMC article. Updated.
-
Topical treatment for facial burns.Cochrane Database Syst Rev. 2020 Jul 29;7(7):CD008058. doi: 10.1002/14651858.CD008058.pub3. Cochrane Database Syst Rev. 2020. PMID: 32725896 Free PMC article.
References
-
- Peng W., Li D., Dai K., et al. Recent progress of collagen, chitosan, alginate and other hydrogels in skin repair and wound dressing applications. Int J Biol Macromol. 2022;208:400–408. - PubMed
-
- Fu X., Sun X., Li X., et al. Dedifferentiation of epidermal cells to stem cells in vivo. Lancet. 2001;358(9287):1067–1068. - PubMed
-
- Viswanathan V., Juttada U., Babu M. Efficacy of recombinant human epidermal growth factor (Regen-D 150) in healing diabetic foot ulcers: A hospital-based randomized controlled trial. Int J Low Extrem Wounds. 2020;19(2):158–164. - PubMed
LinkOut - more resources
Full Text Sources

