Organosulfates from Dark Aqueous Reactions of Isoprene-Derived Epoxydiols Under Cloud and Fog Conditions: Kinetics, Mechanism, and Effect of Reaction Environment on Regioselectivity of Sulfate Addition
- PMID: 40290487
- PMCID: PMC12029931
- DOI: 10.1021/acsearthspacechem.0c00293
Organosulfates from Dark Aqueous Reactions of Isoprene-Derived Epoxydiols Under Cloud and Fog Conditions: Kinetics, Mechanism, and Effect of Reaction Environment on Regioselectivity of Sulfate Addition
Abstract
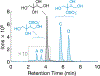
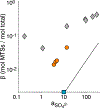
Atmospheric oxidation of isoprene yields large quantities of highly water-soluble isoprene epoxydiols (IEPOX) that partition into fogs, clouds, and wet aerosols. In aqueous aerosols, acid-catalyzed ring-opening of IEPOX followed by nucleophilic addition of inorganic sulfate or water forms organosulfates and 2-methyltetrols, respectively, contributing substantially to secondary organic aerosol (SOA). However, the fate of IEPOX in clouds, fogs and evaporating hydrometeors is not well understood. Here we investigate the rates, product branching ratios, and stereochemistry of organosulfates from reactions of dilute IEPOX (5 to 10 mM) under a range of sulfate concentrations (0.3 to 50 mM) and pH values (1.83-3.38) in order to better understand the fate of IEPOX in clouds and fogs. From these aqueous dark reactions of β-IEPOX isomers (trans- and cis-2-methyl-2,3-epoxybutane-1,4-diols), which are the predominant IEPOX isomers, products were identified and quantified using hydrophilic interaction liquid chromatography coupled to an electrospray ionization high-resolution quadrupole time-of-flight mass spectrometer operated in negative ion mode (HILIC/(-)ESI-HR-QTOFMS). We found that regiochemistry and stereochemistry were affected by pH and the tertiary methyltetrol sulfate (C5H12O7S) was promoted by increasing solution acidity. Furthermore, the rate constants for the reaction of IEPOX under cloud-relevant conditions are up to one order of magnitude lower than reported in the literature for aerosol-relevant conditions due to markedly different solution activity. Nevertheless, the contribution of cloud and fog water reactions to IEPOX SOA may be significant in cases of lower aqueous-phase pH (model estimate) or during droplet evaporation (not studied).
Keywords: 2-methyl-2,3-epoxybutane-1,4-diols; 2-methyltetrols; Biogenic-anthropogenic interaction; IEPOX; Molecular tracers; Multiphase chemistry; Stereochemistry; aqSOA.
Figures



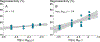

References
-
- IPCC, 2013: Summary for Policymakers. In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V and Midgley PM (Eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
- Clim. Change 2013 Phys. Sci. Basis Contrib. Work. Group Fifth Assess. Rep. Intergov. Panel Clim. Change 2013.
-
- Kreidenweis SM; Petters M; Lohmann U 100 Years of Progress in Cloud Physics, Aerosols, and Aerosol Chemistry Research. Meteorol. Monogr 2018, 59, 11.1–11.72. 10.1175/AMSMONOGRAPHS-D-18-0024.1. - DOI
-
- West JJ; Cohen A; Dentener F; Brunekreef B; Zhu T; Armstrong B; Bell ML; Brauer M; Carmichael G; Costa DL; Dockery DW; Kleeman M; Krzyzanowski M; Künzli N; Liousse C; Lung S-CC; Martin RV; Pöschl U; Pope CA; Roberts JM; Russell AG; Wiedinmyer C “What We Breathe Impacts Our Health: Improving Understanding of the Link between Air Pollution and Health”. Environ. Sci. Technol 2016, 50 (10), 4895–4904. 10.1021/acs.est.5b03827. - DOI - PMC - PubMed
-
- Zhang Q; Jimenez JL; Canagaratna MR; Allan JD; Coe H; Ulbrich I; Alfarra MR; Takami A; Middlebrook AM; Sun YL; Dzepina K; Dunlea EJ; Docherty KS; DeCarlo PF; Salcedo D; Onasch T; Jayne JT; Miyoshi T; Shimono A; Hatakeyama S; Takegawa N; Kondo Y; Schneider J; Drewnick F; Borrmann S; Weimer S; Demerjian K; Williams P; Bower K; Bahreini R; Cottrell L; Griffin RJ; Rautiainen J; Sun JY; Zhang YM; Worsnop DR Ubiquity and Dominance of Oxygenated Species in Organic Aerosols in Anthropogenically-Influenced Northern Hemisphere Midlatitudes. Geophys. Res. Lett 2007, 34 (13), L13801. 10.1029/2007GL029979. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
