Treatable Traits in Patients with Obstructive Lung Diseases in a Well-Established Asthma/COPD Service for Primary Care
- PMID: 40290584
- PMCID: PMC12034284
- DOI: 10.2147/COPD.S508281
Treatable Traits in Patients with Obstructive Lung Diseases in a Well-Established Asthma/COPD Service for Primary Care
Abstract
Purpose: The primary objective of this study was to assess the prevalence of treatable traits (TTs) in patients with obstructive lung diseases in a primary care setting and how these TTs co-occur. The secondary objective was to assess the stability of TTs and the effect of management advice on changes in traits and health outcomes.
Patients and methods: Data from the Dutch asthma/COPD service (2007-2023) were studied retrospectively. Patients ≥18 years with asthma, COPD, or Asthma-COPD overlap (ACO) were included. The prevalence of eight TTs were assessed: 1) insufficient inhaler technique, 2) poor medication adherence, 3) blood eosinophilia, 4) smoking, 5) obesity, 6) physical inactivity, 7) reversible airflow limitation, and 8) anxiety and/or depression. The effect of management advice on TTs was evaluated for patients with a follow-up visit scheduled within 1-2 years.
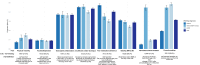
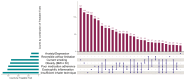
Results: In total, 15246 patients (COPD n=4822; ACO n=1761, asthma n=8663) were included. The highest proportions of TTs were insufficient inhaler technique: 43.6% (95% CI: 42.9-44.4), followed by poor medication adherence: 40.3% (95% CI: 39.2-41.4) and blood eosinophilia: 36.9% (95% CI: 35.8-38.1). Overall, 83.3% of patients had ≥ 1 TTs, and 48.9% of patients ≥ 2 TTs. Among patients with blood eosinophilia, a significant reduction of the trait at follow-up (OR: 0.61, 95% CI: 0.39; 0.96) and improved health status were observed when the pulmonologist advised the general practitioner to initiate or increase the dose of ICS. No significant association was found between management advice and the exacerbation rate at follow-up.
Conclusion: The TTs assessed in this study are common in primary care patients, with nearly half of the patients showing a combination of at least two TTs. These TTs coexist in many different combinations. A personalized approach targeting these traits may be effective in achieving better control of these heterogeneous diseases.
Keywords: COPD; asthma; asthma-COPD overlap; precision medicine; treatable traits.
© 2025 Dijk et al.
Conflict of interest statement
LD, YG are employed by the General Practitioners Research Institute (GPRI). In the past three years (2022-2024), GPRI conducted investigator- and sponsor-initiated research funded by non-commercial organizations, academic institutes, and pharmaceutical companies (including AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Mundipharma, Novartis, and Teva). JWK reports grants, personal fees and non-financial support from AstraZeneca, grants, personal fees and non-financial support from Boehringer Ingelheim, grants and personal fees from Chiesi, grants, personal fees and non-financial support from GSK, non-financial support from Mundi Pharma, grants and personal fees from Teva, personal fees from MSD, personal fees from COVIS Pharma, personal fees from ALK-Abello, grants from Valneva outside the submitted work; and Janwillem Kocks holds <5% shares of Lothar Medtec GmbH and is owner the General Practitioners Research Institute. TvdM reports personal fees and travel grants from GSK, Chiesi. IP has received speaker’s fees, payments for organizing education events, honoraria for attending advisory panels, sponsorship to attend international scientific meetings, research grants or payments to support FDA approval meetings from Aerocrine, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Roche-Genentech, GSK, Knopp, Merck, Novartis, Sanofi-Regeneron, and Teva; acted as an expert witness for a patent dispute involving AstraZeneca and Teva; is a co-patent holder for the Leicester Cough Questionnaire, and received payments for use of the Leicester Cough Questionnaire in clinical trials from Bayer, Insmed, and Merck. IP is a member of the editorial board of the International journal of Chronic Obstructive Pulmonary Disease. HAMK has performed consultancies for, received unrestricted research and educational grants from, and has participated in clinical trials on a per patient fee basis for: AstraZeneca, Boehringer Ingelheim, Chiesi, GSK and Novartis. All renumerations were non personal and were received by his institution. The authors report no other conflicts of interest in this work.
Figures




References
-
- 2024 GINA main report - global initiative for asthma - GINA. Available from: https://ginasthma.org/2024-report/. Accessed September 20, 2024.
-
- 2024 GOLD report - global initiative for chronic obstructive lung disease - GOLD. Available from: https://goldcopd.org/2024-gold-report/. Accessed September 20, 2024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

