Synthesis, molecular docking, and biological investigations of new pyrazolone chalcones
- PMID: 40290744
- PMCID: PMC12023741
- DOI: 10.1039/d5ra01233c
Synthesis, molecular docking, and biological investigations of new pyrazolone chalcones
Abstract
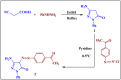
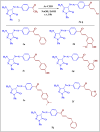
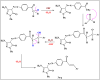
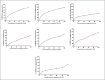
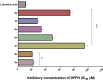
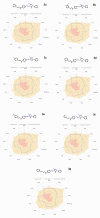
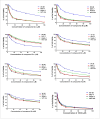
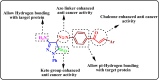
Heterocyclic compounds are essential to the drug development and discovery processes. Herein, we synthesized new pyrazolone chalcones (3a-g) through the reaction of azopyrazolone (2) with different aromatic aldehydes in a basic medium. Numerous techniques including elemental analysis, 1H-NMR, 13C-NMR, and FT-IR spectroscopies, were used to characterize pyrazolone chalcone derivatives. Compound 3b exhibited the highest binding energy towards YAP/TEAD protein with a value of -8.45 kcal mol-1 in in silico studies. This observation suggested that compound 3b inhibits the YAP/TEAD Hippo signaling pathway. In addition, compound 3b offered a prospective anticancer effect against various cancer cell lines, such as HepG-2, MCF-7, and HCT-116, among the other synthesized compounds, with IC50 values equal to 5.03 ± 0.4, 3.92 ± 0.2, and 6.34 ± 0.5 μM, respectively. These results validated our findings regarding the in silico suppression of the YAP/TEAD protein. Its pharmacokinetic properties were theoretically observed using ADMET. Additionally, compound 3b demonstrated a potent antioxidant scavenging action (in vitro) against DPPH free radicals. Thus, based on our findings, compound 3b may act as a potential anticancer scaffold owing to its inhibitory impact towards the YAP/TEAD-mediated Hippo signaling pathway with a safe toxic profile on normal cells.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures

References
-
- Das P. Sayan G. Tarun A. Pritiprasanna M. Sabyasachi G. Sumita C. Subhashis G. Mater. Chem. Phys. 2019;237:121860.
-
- Zhao Y. Yang X. Int. J. Cancer. 2015;137:2767–2773. - PubMed
-
- Patil J. V. Shubhangi S. S. Shweta U. Pankaj G. Suresh B. ChemistrySelect. 2024;9:e202303391.
LinkOut - more resources
Full Text Sources

