Exploring the Therapeutic Mechanism of Jianpi Zhidong Decoction on Tourette Syndrome Based on Proteomics and Network Pharmacology
- PMID: 40291161
- PMCID: PMC12034289
- DOI: 10.2147/DDDT.S505173
Exploring the Therapeutic Mechanism of Jianpi Zhidong Decoction on Tourette Syndrome Based on Proteomics and Network Pharmacology
Abstract
Purpose: To explore the pharmacological effects of Jianpi Zhidong Decoction (JPZDD) on Tourette Syndrome (TS) using proteomics and network pharmacology.
Materials and methods: Chemical components of JPZDD were identified via UPLC-MS/MS. Chronic restraint stress TS model was established by intraperitoneal injection of iminodipropionitrile (IDPN) for 1 week with restraint stress for 3 weeks. Sixty male SD rats were divided into control, model, Tiapride (Tia), and JPZDD groups. After the intervention of 28 days, behavioral tests, Nissl staining, Western blot, immunofluorescence, colorimetry, and ELISA were performed to evaluate the pharmacological effects of JPZDD. Proteomics and network pharmacology predicted targets, validated by Western blot.
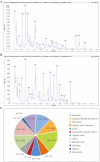
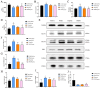
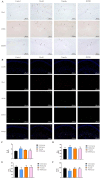
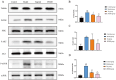
Results: JPZDD alleviated stereotypic behaviors, hippocampal pathology, and modulated glucose metabolites (GLU, pyruvate, lactate, ATP). It downregulated GLUT1, GLUT3, HK2, and LDHA levels while upregulating PDHA level. Besides, JPZDD balanced M1/M2 microglial phenotypes, reducing IL-1β and IL-6 and increasing IL-4 and IL-10. UPLC-MS/MS identified 44 active ingredients and 123 targets; proteomics revealed 28 differentially expressed proteins. GO/KEGG analysis implicated that the PI3K/AKT/mTOR pathway may be the molecular target. JPZDD inhibited PI3K, AKT, and mTOR phosphorylation.
Conclusion: JPZDD (16 g·kg⁻¹·d⁻¹) alleviates motor tics, modulates microglial activation and glucose metabolism, and downregulates the PI3K/AKT/mTOR pathway, providing a mechanistic basis for its therapeutic role in TS.
Keywords: Jianpi Zhidong decoction; Tourette syndrome; glucose metabolism; microglia; network pharmacology.
© 2025 Zhang et al.
Conflict of interest statement
The authors have declared that no competing interests exist in this work.
Figures







Similar articles
-
Network Pharmacology Combined with Animal Models to Investigate the Mechanism of ChangPu YuJin Tang in the Treatment of Tourette Syndrome.Comb Chem High Throughput Screen. 2025;28(1):166-184. doi: 10.2174/0113862073295447240430113053. Comb Chem High Throughput Screen. 2025. PMID: 38706359 Free PMC article.
-
Jian-Pi-Zhi-Dong-Decoction regulates the expression of glutamate transporters to attenuate glutamate excitotoxicity and exerts anti-tics effects in Tourette syndrome model rats.Neuropsychiatr Dis Treat. 2018 Dec 17;14:3381-3392. doi: 10.2147/NDT.S185169. eCollection 2018. Neuropsychiatr Dis Treat. 2018. PMID: 30587990 Free PMC article.
-
Effects of Jian-Pi-Zhi-Dong Decoction on the Expression of 5-HT and Its Receptor in a Rat Model of Tourette Syndrome and Comorbid Anxiety.Med Sci Monit. 2020 Aug 1;26:e924658. doi: 10.12659/MSM.924658. Med Sci Monit. 2020. PMID: 32738135 Free PMC article.
-
Elucidating the mechanisms of Buyang Huanwu Decoction in treating chronic cerebral ischemia: A combined approach using network pharmacology, molecular docking, and in vivo validation.Phytomedicine. 2024 Sep;132:155820. doi: 10.1016/j.phymed.2024.155820. Epub 2024 Jun 24. Phytomedicine. 2024. PMID: 39004032
-
Effect of Jian-Pi-Zhi-Dong Decoction on striatal glutamate and γ-aminobutyric acid levels detected using microdialysis in a rat model of Tourette syndrome.Neuropsychiatr Dis Treat. 2016 May 19;12:1233-42. doi: 10.2147/NDT.S106330. eCollection 2016. Neuropsychiatr Dis Treat. 2016. PMID: 27279743 Free PMC article.
Cited by
-
Network Pharmacology-Driven Sustainability: AI and Multi-Omics Synergy for Drug Discovery in Traditional Chinese Medicine.Pharmaceuticals (Basel). 2025 Jul 21;18(7):1074. doi: 10.3390/ph18071074. Pharmaceuticals (Basel). 2025. PMID: 40732361 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

