Causes, Diagnosis, Treatment, and Prognosis of Cardiac Fibrosis: A Systematic Review
- PMID: 40291288
- PMCID: PMC12032538
- DOI: 10.7759/cureus.81264
Causes, Diagnosis, Treatment, and Prognosis of Cardiac Fibrosis: A Systematic Review
Abstract
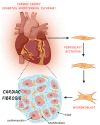
Cardiac fibrosis, characterized by excessive extracellular matrix deposition, contributes to heart failure, arrhythmias, and myocardial dysfunction. Despite advances in understanding its mechanisms, targeted antifibrotic therapies remain limited. This review examines the causes, molecular mechanisms, diagnostic approaches, and therapeutic strategies for cardiac fibrosis. A systematic review of peer-reviewed studies was conducted, focusing on the etiology, diagnosis, treatment, and prognosis of cardiac fibrosis with no specific timeframe. The condition is driven by fibroblast activation, inflammatory pathways, and mechanical stress, with key contributing factors including ischemic heart disease, hypertension, diabetes, and aging. Diagnostic tools such as cardiac magnetic resonance imaging with T1 mapping and biomarkers play a crucial role, with natriuretic peptides offering both diagnostic and prognostic value. Galectin-3 has also shown promise as a prognostic marker. Current therapies, including RAAS inhibitors and beta-blockers, help prevent fibrosis progression but do not reverse established fibrosis. Emerging strategies such as plant-based compounds, gene therapy, fibroblast-targeting vaccines, and stem cell reprogramming show potential in preclinical studies. However, cardiac fibrosis remains a major driver of heart disease progression, and existing treatments remain limited. Major gaps include the lack of validated antifibrotic agents and challenges in translating preclinical findings into clinical applications. Further research is essential to develop effective targeted interventions.
Keywords: antifibrotic therapies; cardiac biomarker; cardiac fibrosis; fibroblast activation; heart failure.
Copyright © 2025, BaniHani et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
Similar articles
-
Cardiac Fibrosis in heart failure: Focus on non-invasive diagnosis and emerging therapeutic strategies.Mol Aspects Med. 2023 Oct;93:101194. doi: 10.1016/j.mam.2023.101194. Epub 2023 Jun 27. Mol Aspects Med. 2023. PMID: 37384998 Review.
-
Emerging Epigenetic Therapies for the Treatment of Cardiac Fibrosis.Biomedicines. 2025 May 11;13(5):1170. doi: 10.3390/biomedicines13051170. Biomedicines. 2025. PMID: 40426997 Free PMC article. Review.
-
Bioactive Compounds and Cardiac Fibrosis: Current Insight and Future Prospect.J Cardiovasc Dev Dis. 2023 Jul 21;10(7):313. doi: 10.3390/jcdd10070313. J Cardiovasc Dev Dis. 2023. PMID: 37504569 Free PMC article. Review.
-
Targeting GPCRs to treat cardiac fibrosis.Front Cardiovasc Med. 2022 Oct 6;9:1011176. doi: 10.3389/fcvm.2022.1011176. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36277752 Free PMC article. Review.
-
Activated fibroblasts in cardiac and cancer fibrosis: An overview of analogies and new potential therapeutic options.Life Sci. 2023 May 15;321:121575. doi: 10.1016/j.lfs.2023.121575. Epub 2023 Mar 16. Life Sci. 2023. PMID: 36933828 Review.
Cited by
-
Longitudinal Myocardial Deformation as an Emerging Biomarker for Post-Traumatic Cardiac Dysfunction.Life (Basel). 2025 Jun 30;15(7):1052. doi: 10.3390/life15071052. Life (Basel). 2025. PMID: 40724554 Free PMC article. Review.
References
-
- Cardiac fibrosis: cell biological mechanisms, molecular pathways and therapeutic opportunities. Frangogiannis NG. Mol Aspects Med. 2019;65:70–99. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
