Obesity accelerates age-related memory deficits and alters white matter tract integrity in Ldlr-/-.Leiden mice
- PMID: 40291340
- PMCID: PMC12032874
- DOI: 10.1016/j.bbih.2025.100991
Obesity accelerates age-related memory deficits and alters white matter tract integrity in Ldlr-/-.Leiden mice
Abstract
Background: Obesity in mid-adulthood has been suggested to promote brain aging and is associated with progressive cognitive impairment later in life. However, the structural and functional alterations that underlie obesity-related cognitive dysfunction are still poorly understood, partly owing to the lack of translational models replicating age- and obesity-related brain pathology.
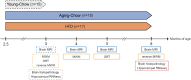
Methods: The effect of age and high-fat diet (HFD)-induced obesity was investigated in adult Ldlr-/-.Leiden mice, an established translational model for obesity and its comorbidities. During mid-adulthood, from three to eight months of age, brain structure and function (hippocampal volume, cortical thickness, white matter integrity, cerebral blood flow (CBF), resting-state functional connectivity) were monitored with brain magnetic resonance imaging, and cognitive function was evaluated using cognitive tests. Brain pathology was further examined with histopathological and gene expression analyses.
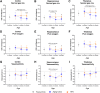
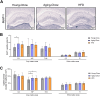
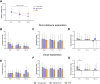
Results: Ldlr-/-.Leiden mice showed age-related decreases in cortical thickness, CBF, brain connectivity, and neurogenesis along with the development of neuroinflammation and (short-term) memory impairments. On HFD feeding, Ldlr-/-.Leiden mice exhibited similar features, but memory deficits started at a younger age than in chow-fed mice. HFD-fed mice additionally showed a rise in CBF with concomitant decline in fractional anisotropy in white matter tracts. Analyses of hippocampal gene expression further revealed an age-related suppression of processes related to metabolic and neuronal function while HFD feeding strongly activated neuroinflammatory pathways.
Conclusions: Ldlr-/-.Leiden mice show similar critical age-related changes in brain structure and function as observed in humans. In this mouse model, HFD feeding particularly trigger disturbances in brain blood perfusion and white matter tract integrity, which may underlie an accelerated cognitive decline in obesity.
Keywords: Aging; Cognitive impairment; Neurodegeneration; Neuroimaging; Obesity.
© 2025 The Authors. Published by Elsevier Inc.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Arnoldussen I.A.C., Wiesmann M., Pelgrim C.E., Wielemaker E.M., Van Duyvenvoorde W., Amaral-Santos P.L., Verschuren L., Keijser B.J.F., Heerschap A., Kleemann R., Wielinga P.Y., Kiliaan A.J. Butyrate restores HFD-induced adaptations in brain function and metabolism in mid-adult obese mice. Int. J. Obes. 2017;41:935–944. doi: 10.1038/ijo.2017.52. - DOI - PubMed
-
- Arnoldussen I.A.C., Morrison M.C., Wiesmann M., van Diepen J.A., Worms N., Voskuilen M., Verweij V., Geenen B., Gualdo N.P., van der Logt L., Gross G., Kleemann R., Kiliaan A.J. Milk fat globule membrane attenuates high fat diet-induced neuropathological changes in obese Ldlr−/−.Leiden mice. Int. J. Obes. 2022;46:342–349. doi: 10.1038/s41366-021-00998-w. - DOI - PubMed
LinkOut - more resources
Full Text Sources

