The Barcelona baseline risk score to predict long-term prognosis after a first demyelinating event: a prospective observational study
- PMID: 40291402
- PMCID: PMC12033939
- DOI: 10.1016/j.lanepe.2025.101302
The Barcelona baseline risk score to predict long-term prognosis after a first demyelinating event: a prospective observational study
Abstract
Background: In multiple sclerosis (MS), predicting at symptom onset who will develop early and severe disability is an unmet need with significant therapeutic implications. Here we propose the Barcelona-Baseline Risk Score (BRS) model to predict long-term disease outcomes in a flexible and generalisable manner.
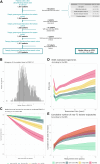
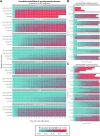
Methods: Using prospectively acquired data from the Barcelona first-attack cohort, we created the Barcelona-BRS model as a set of six Weibull survival models of time to an Expanded Disability Status Scale score of 3.0, built with flexible combinations of predictors, including sex, age at first attack, and number and topography of T2 lesions, among others, adaptable to data availability. Data-driven risk groups were identified and compared in terms of long-term clinical and MRI outcomes, including relapse-associated worsening (RAW), progression independent of relapse activity (PIRA), conversion to secondary progressive MS (SPMS), lesional and brain volumetric data, and patient-reported/administered clinical scores, through Kaplan-Meier and mixed-effects models. Finally, we externally validated our model in a completely unseen cohort.
Findings: We included 1074 patients (737 [69%] female, mean age: 31.7 years) with a first demyelinating attack. Over a median follow-up of 11.9 years, 375 (35%), 298 (28%), and 94 (8.8%) developed RAW, PIRA, and SPMS, respectively. Weibull models included age at first attack, number of brain T2 lesions, and disability at first visit as main predictors. Four data-driven groups of increasing risk of unfavourable outcomes were created: Light-Green-BRS (N = 258), Dark-Green-BRS (N = 319), Orange-BRS (N = 321), and Red-BRS (N = 176), which, over time, behaved significantly differently across disability, quality of life, and MRI measures, being the Red-BRS the group with worst outcomes (p < 0.01). The results in the external validation cohort (N = 139, 100 female [72%], 34 years) mirrored those of the original one.
Interpretation: The robustness, flexibility, and generalisability of the Barcelona-BRS model support its consideration as a ready-to-use tool for clinical practice.
Funding: None.
Keywords: Disease progression; MRI; Multiple sclerosis; Predictive model; Progression independent of relapse activity; Relapse-associated worsening.
© 2025 The Authors.
Conflict of interest statement
C. Tur is currently being funded by a Miguel Servet contract, awarded by the Instituto de Salud Carlos III (ISCIII), Ministerio de Ciencia e Innovación de España (CP23/00117). She has also received a 2020 Junior Leader La Caixa Fellowship (fellowship code: LCF/BQ/PI20/11760008), awarded by “la Caixa” Foundation (ID100010434), a 2021 Merck's Award for the Investigation in MS, awarded by Fundación Merck Salud (Spain), 2021 and 2024 Research Project Grants (PI21/01860 and PI24/01277) awarded by the ISCIII, Ministerio de Ciencia e Innovación de España, and a FORTALECE research grant (FORT23/00034) also by the ISCIII, Ministerio de Ciencia e Innovación de España. In 2015, she received an ECTRIMS Post-doctoral Research Fellowship and has received funding from the UK MS Society. She is a member of the Editorial Board of Neurology Journal and Multiple Sclerosis Journal. She has also received honoraria from Roche, Novartis, Merck, Sanofi, Immunic Therapeutics, and Bristol Myers Squibb. She is a steering committee member of the O’HAND trial (Roche) and of the Consensus group on Follow-on DMTs. P. Carbonell-Mirabent has nothing to disclose. S. Otero-Romero has received speaking and consulting honoraria from Genzyme, Biogen-Idec, Novartis, Roche, Excemed and MSD; as well as research support from Novartis. A. Cobo-Calvo has received grant from Instituto de Salud Carlos III, Spain; JR19/00007. M.J. Arévalo has nothing to disclose. H. Ariño has nothing to disclose. G. Arrambide has received compensation for consulting services, speaking honoraria or participation in advisory boards from Merck, Roche, and Horizon Therapeutics; and travel support for scientific meetings from Novartis, Roche, ECTRIMS and EAN. Has received speaking honoraria and consulting services or participation in advisory boards from Sanofi, Merck, Roche and Horizon Therapeutics; travel expenses for scientific meetings from Novartis, Roche, and ECTRIMS. C. Auger has nothing to disclose. R. Carvajal is currently being funded by a Research grand from the European Charcot Foundation. In 2023 he received a grand by “Vall d'Hebron Institut de Recerca”. In 2021, he received an ECTRIMS Fellowship training performed during 2021–2022. He has also received speaking honoraria from Roche, Novartis, Biogen, Merck and Sanofi. J. Castilló has nothing to disclose. M. Comabella has received compensation for consulting services and speaking honoraria from Bayer Schering Pharma, Merck Serono, Biogen-Idec, Teva Pharmaceuticals, Sanofi-Aventis, Genzyme, and Novartis. I. Galán has nothing to disclose. L. Midaglia has nothing to disclose. C. Nos has nothing to disclose. A. Pappolla has received funding travel from Roche and speaking honoraria from Novartis. He performed an ECTRIMS Clinical Training Fellowship program during 2021 and is currently performing an MSIF-ARSEP Fellowship program. D. Pareto holds a research grant from the Instituto Carlos III (PI22/01709), co-funded by the European Union. J. Río has received speaking honoraria and personal compensation for participating on Advisory Boards from Biogen-Idec, Genzyme, Janssen, Merck- Serono, Novartis, Teva, Roche, and Sanofi-Aventis. B. Rodríguez-Acevedo has received speaking honoraria from Merck and honoraria for consulting services from Novartis. E. Saez de Gordoa has nothing to disclose. A. Vidal-Jordana has received support has received support for contracts Juan Rodes (JR16/00024) and from Fondo de Investigación en Salud (PI17/02162 and PI22/01589) from Instituto de Salud Carlos III, Spain, and has engaged in consulting and/or participated as speaker in events organized by Roche, Novartis, and Merck. A. Zabalza has received a Rio Hortega grant, from the Instituto de Salud Carlos III, Spain (CM22/00237) and received travel expenses for scientific meetings from Biogen-Idec, Merck Serono and Novartis; speaking honoraria from Eisai; and a study grant from Novartis. J. Sastre-Garriga serves as co-Editor for Europe on the editorial board of Multiple Sclerosis Journal and as Editor-in-Chief in Revista de Neurología, receives research support from Fondo de Investigaciones Sanitarias (19/950) and has served as a consultant/speaker for Biogen, Celgene/Bristol Meyers Squibb, Sanofi, Novartis and Merck. À. Rovira serves on scientific advisory boards for Novartis, Sanofi-Genzyme, Synthetic MR, Roche, Biogen, and OLEA Medical; has received speaker honoraria from Bayer, SanofiGenzyme, Merck-Serono, Teva Pharmaceutical Industries Ltd, Novartis, Roche, and Biogen; and is CMO and co-founder of TensorMedical. X. Montalban has received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Abbvie, Actelion, Alexion, Biogen, Bristol-Myers Squibb/Celgene, EMD Serono, Genzyme, Hoffmann-La Roche, Immunic, Janssen Pharmaceuticals, Medday, Merck, Mylan, Nervgen, Novartis, Sandoz, Sanofi-Genzyme, Teva Pharmaceutical, TG Therapeutics, Excemed, MSIF and NMSS. M. Tintoré has received compensation for consulting services, speaking honoraria and research support from Almirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Immunic Therapeutics, Janssen, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Viela Bioand Teva Pharmaceuticals. Data Safety Monitoring Board for Parexel and UCB Biopharma, Relapse Adjudication Committee for IMCYSE SA.
Figures


References
-
- Thompson A.J., Baranzini S.E., Geurts J., Hemmer B., Ciccarelli O. Multiple sclerosis. Lancet. 2018;391:1622–1636. - PubMed
-
- Jakimovski D., Bittner S., Zivadinov R., et al. Multiple sclerosis. Lancet. 2024;403:183–202. - PubMed
-
- Kappos L., Wolinsky J.S., Giovannoni G., et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132–1140. - PMC - PubMed
LinkOut - more resources
Full Text Sources

