This is a preprint.
Beta Adrenergic Signaling as a Therapeutic Target for Autoimmunity
- PMID: 40291744
- PMCID: PMC12026814
- DOI: 10.1101/2025.04.05.647384
Beta Adrenergic Signaling as a Therapeutic Target for Autoimmunity
Update in
-
Beta adrenergic signaling as a therapeutic target for autoimmunity.J Neuroimmunol. 2025 Oct 15;407:578705. doi: 10.1016/j.jneuroim.2025.578705. Epub 2025 Jul 24. J Neuroimmunol. 2025. PMID: 40729967 Free PMC article.
Abstract
Background: We recently identified a molecular mechanism involving beta-adrenergic 1 and 2 receptors (β1/2) in the development of TH17 lymphocytes. Pharmacological and genetic inhibition of these receptors in combination, but not separately, impaired the ability of T-lymphocytes to produce proinflammatory interleukin 17A (IL-17A) and instead promoted the production of protective Treg cells that secrete anti-inflammatory interleukin-10 (IL-10). However, it remained unclear whether this regulatory mechanism could serve as a novel therapeutic approach for autoimmune disorders mediated by IL-17A-producing T-lymphocytes.
Methods: Multiple sclerosis (MS) is an inflammatory demyelinating disorder of the central nervous system (CNS) characterized by an autoimmune response where both T-lymphocytes and IL-17A are implicated in the pathogenesis of the disease. Using an animal model of MS, termed experimental autoimmune encephalomyelitis (EAE), we addressed the impact of beta adrenergic receptor blockade (genetically and pharmacologically) on EAE disease progression, severity, and TH17/Treg balance.
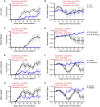
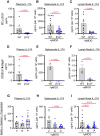
Results: The genetic deletion β1/2 receptors, either systemically or specifically in T-lymphocytes, significantly attenuated EAE disease severity and animal weight loss. Pharmacological blockade of β1/2 receptors with either propranolol (lipophilic) or nadolol (aqueous) limited disease severity and weight loss similar to the genetic models. All models showed degrees of shifted TH17/Treg balance (suppressing TH17 and promoting Treg) and decreased T-lymphocyte IL-17A production. Importantly, pharmacological blockade was initiated at the time of symptom development, which mimics the typical time where diagnosis of disease would occur.
Conclusions: Our data depict a novel role for β1/2 adrenergic signaling in the control of TH17/Treg cells in EAE. These findings provide new insight into the disease progression as well as provide a potential new pharmacological therapy for IL-17A-related autoimmune diseases.
Keywords: EAE; IL-17A; T-Lymphocyte; autonomic; propranolol.
Conflict of interest statement
Conflict of Interest Statement: The authors have declared that no conflict of interest exists.
Figures

References
-
- Felten DL, Livnat S, Felten SY, Carlson SL, Bellinger DL, and Yeh P. Sympathetic innervation of lymph nodes in mice. Brain Res Bull. 1984;13(6):693–9. - PubMed
-
- Felten DL, Felten SY, Carlson SL, Olschowka JA, and Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135(2 Suppl):755s-65s. - PubMed
-
- Giron LT, Crutcher KA, and Davis JN. Lymph nodes--a possible site for sympathetic neuronal regulation of immune responses. Ann Neurol. 1980;8(5):520–5. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
