Magnetic resonance imaging bias field correction improves tumor prognostic evaluation after transcatheter arterial chemoembolization for liver cancer
- PMID: 40291888
- PMCID: PMC12019036
- DOI: 10.4240/wjgs.v17.i4.104187
Magnetic resonance imaging bias field correction improves tumor prognostic evaluation after transcatheter arterial chemoembolization for liver cancer
Abstract
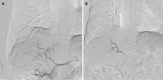
Background: Transcatheter arterial chemoembolization (TACE) is a key treatment approach for advanced invasive liver cancer (infiltrative hepatocellular carcinoma). However, its therapeutic response can be difficult to evaluate accurately using conventional two-dimensional imaging criteria due to the tumor's diffuse and multifocal growth pattern. Volumetric imaging, especially enhanced tumor volume (ETV), offers a more comprehensive assessment. Nonetheless, bias field inhomogeneity in magnetic resonance imaging (MRI) poses challenges, potentially skewing volumetric measurements and undermining prognostic evaluation.
Aim: To investigate whether MRI bias field correction enhances the accuracy of volumetric assessment of infiltrative hepatocellular carcinoma treated with TACE, and to analyze how this improved measurement impacts prognostic prediction.
Methods: We retrospectively collected data from 105 patients with invasive liver cancer who underwent TACE treatment at the Affiliated Hospital of Xuzhou Medical University from January 2020 to January 2024. The improved N4 bias field correction algorithm was applied to process MRI images, and the ETV before and after treatment was calculated. The ETV measurements before and after correction were compared, and their relationship with patient prognosis was analyzed. A Cox proportional hazards model was used to evaluate prognostic factors, with Martingale residual analysis determining the optimal cutoff value, followed by survival analysis.
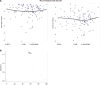
Results: Bias field correction significantly affected ETV measurements, with the corrected baseline ETV mean (505.235 cm³) being significantly lower than before correction (825.632 cm³, P < 0.001). Cox analysis showed that the hazard ratio (HR) for corrected baseline ETV (HR = 1.165, 95%CI: 1.069-1.268) was higher than before correction (HR = 1.063, 95%CI: 1.031-1.095). Using 412 cm³ as the cutoff, the group with baseline ETV < 415 cm³ had a longer median survival time compared to the ≥ 415 cm³ group (18.523 months vs 8.926 months, P < 0.001). The group with an ETV reduction rate ≥ 41% had better prognosis than the < 41% group (17.862 months vs 9.235 months, P = 0.006). Multivariate analysis confirmed that ETV reduction rate (HR = 0.412, P < 0.001), Child-Pugh classification (HR = 0.298, P < 0.001), and Barcelona Clinic Liver Cancer stage (HR = 0.578, P = 0.045) were independent prognostic factors.
Conclusion: Volume imaging based on MRI bias field correction can improve the accuracy of evaluating the efficacy of TACE treatment for invasive liver cancer. The corrected ETV and its reduction rate can serve as independent indicators for predicting patient prognosis, providing important reference for developing individualized treatment strategies.
Keywords: Bias field correction; Invasive liver cancer; Magnetic resonance imaging; Transcatheter arterial chemoembolization; Volume imaging.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures

Similar articles
-
Predicting Infiltrative Hepatocellular Carcinoma Patient Outcome Post-TACE: MR Bias Field Correction Effect on 3D-quantitative Image Analysis.J Clin Transl Hepatol. 2020 Sep 28;8(3):292-298. doi: 10.14218/JCTH.2020.00054. Epub 2020 Aug 18. J Clin Transl Hepatol. 2020. PMID: 33083252 Free PMC article.
-
5-Fluorouracil combined with CalliSphere drug-eluting beads or conventional transarterial chemoembolization for unresectable hepatocellular carcinoma: a propensity score weighting analysis.Sci Rep. 2024 Oct 26;14(1):25588. doi: 10.1038/s41598-024-77531-2. Sci Rep. 2024. PMID: 39462077 Free PMC article.
-
3D Quantitative tumour burden analysis in patients with hepatocellular carcinoma before TACE: comparing single-lesion vs. multi-lesion imaging biomarkers as predictors of patient survival.Eur Radiol. 2016 Sep;26(9):3243-52. doi: 10.1007/s00330-015-4168-3. Epub 2016 Jan 13. Eur Radiol. 2016. PMID: 26762942 Free PMC article.
-
Identifying enhancement-based staging markers on baseline MRI in patients with colorectal cancer liver metastases undergoing intra-arterial tumor therapy.Eur Radiol. 2021 Dec;31(12):8858-8867. doi: 10.1007/s00330-021-08058-7. Epub 2021 Jun 1. Eur Radiol. 2021. PMID: 34061209 Free PMC article.
-
Transcatheter arterial chemoembolization for infiltrative hepatocellular carcinoma: clinical safety and efficacy and factors influencing patient survival.Korean J Radiol. 2014 Jul-Aug;15(4):464-71. doi: 10.3348/kjr.2014.15.4.464. Epub 2014 Jul 9. Korean J Radiol. 2014. PMID: 25053906 Free PMC article.
References
-
- Ekanayaka SPN, Luke N, Thilakarathne SB, Dassanayake A, Gunetilleke MB, Niriella MA, Siriwardana RC. Characteristics and survival of advanced untreated hepatocellular carcinoma of non-viral etiology. Indian J Gastroenterol. 2024;43:1176–1183. - PubMed
-
- Sun R, Gou Y, Pan L, He Q, Zhou Y, Luo Y, Wu C, Zhao Y, Fu Z, Huang P. Hepatic arterial infusion chemotherapy (HAIC) combined with Tislelizumab and Lenvatinib for unresectable hepatocellular carcinoma: a retrospective single-arm study. Cell Oncol (Dordr) 2024;47:2265–2276. - PubMed
-
- Zhu X, Zhang Z, Zhang J, Xiao Y, Wang H, Wang M, Jiang M, Xu Y. Single-cell and Bulk Transcriptomic Analyses Reveal a Stemness and Circadian Rhythm Disturbance-related Signature Predicting Clinical Outcome and Immunotherapy Response in Hepatocellular Carcinoma. Curr Gene Ther. 2025;25:178–193. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

