Fecal microbiota transplantation alleviates lipopolysaccharide-induced osteoporosis by modulating gut microbiota and long non-coding RNA TUG1 expression
- PMID: 40292220
- PMCID: PMC12021831
- DOI: 10.3389/fcimb.2025.1535666
Fecal microbiota transplantation alleviates lipopolysaccharide-induced osteoporosis by modulating gut microbiota and long non-coding RNA TUG1 expression
Abstract
Purpose: To study whether fecal microbiota transplantation (FMT) can alleviate lipopolysaccharide (LPS)-induced osteoporosis (OP) by regulating the composition and abundance of gut microbiota and the expression level of long non-coding RNA (lncRNA) TUG1.
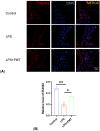
Methods: Twenty C57BL/6 mice were selected. Two mice were randomly designated as fecal donors, while the remaining mice were randomly divided into control group, LPS group, and LPS + FMT group. Each group consisted of 6 mice. The mice in the LPS and LPS + FMT groups were intraperitoneally injected with LPS to establish the OP model, and the mice in the LPS + FMT group were treated with donor feces by gavage. Micro-CT was used to scan the femur specimens of mice, and the bone structural parameters of the control and LPS groups were compared to verify the effectiveness of the OP model. HE staining was used to compare the microstructure of femurs in the 3 groups. 16S rRNA gene sequencing was used to analyze the composition and abundance of gut microbiota in mice. Immunofluorescence staining was used to compare the expression levels of Runt-related transcription factor 2 (RUNX2) in the femur of the 3 groups. Real-time quantitative reverse transcription PCR (qRT-PCR) was used to compare the expression levels of lncRNA TUG1 in the intestines and serum of mice in the 3 groups.
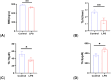
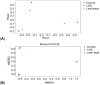
Results: Micro-CT showed that compared with the control group, the mice in the LPS group had more bone loss. The bone mineral density, trabecular number, and trabecular thickness of the control group was higher, and the trabecular separation was smaller. The models were validated effectively. HE staining showed that compared with the control group, the bone trabeculae in the LPS group were thinner and sparse, while that in the LPS + FMT group were dense and clear. The 16s rRNA sequencing showed that the abundance of Bacteroides and Lactobacillus in LPS+FMT group was significantly higher than that in LPS group. Immunofluorescence staining showed that the RUNX2 level in the control group and LPS + FMT group was similar, and both were higher than that in the LPS group. The qRT-PCR results showed that the TUG1 mRNA level in the control group and LPS + FMT group was similar and significantly higher than that in the LPS group.
Conclusion: FMT can enhance osteoblast levels and improve bone structure by modulating the abundance of gut microbiota in OP mice (such as increasing Bacteroides and Lactobacillus populations) and promoting the expression of lncRNA TUG1, thereby alleviating LPS-induced OP.
Keywords: fecal microbiota transplantation; gut microbiota; lipopolysaccharides; lncRNA; osteoporosis.
Copyright © 2025 Ma, Wang, Chen, Zheng, Yang, Meng, Liu, Lu, Zhao and Gao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Fecal microbiota transplantation ameliorates bone loss in mice with ovariectomy-induced osteoporosis via modulating gut microbiota and metabolic function.J Orthop Translat. 2022 Sep 26;37:46-60. doi: 10.1016/j.jot.2022.08.003. eCollection 2022 Nov. J Orthop Translat. 2022. PMID: 36196151 Free PMC article.
-
Fecal microbiota transplantation from postmenopausal osteoporosis human donors accelerated bone mass loss in mice.Front Cell Infect Microbiol. 2024 Dec 5;14:1488017. doi: 10.3389/fcimb.2024.1488017. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39703374 Free PMC article.
-
[Effects and mechanism of fecal transplantation on acute lung injury induced by lipopolysaccharide in rats].Zhonghua Yi Xue Za Zhi. 2019 May 28;99(20):1582-1587. doi: 10.3760/cma.j.issn.0376-2491.2019.20.013. Zhonghua Yi Xue Za Zhi. 2019. PMID: 31154727 Chinese.
-
Fecal microbiota transplantation as a promising treatment option for osteoporosis.J Bone Miner Metab. 2022 Nov;40(6):874-889. doi: 10.1007/s00774-022-01375-x. Epub 2022 Nov 11. J Bone Miner Metab. 2022. PMID: 36357745 Free PMC article. Review.
-
Human-fecal microbiota transplantation in relation to gut microbiome signatures in animal models for schizophrenia: A scoping review.Asian J Psychiatr. 2024 Dec;102:104285. doi: 10.1016/j.ajp.2024.104285. Epub 2024 Oct 24. Asian J Psychiatr. 2024. PMID: 39486191
References
-
- Aquino-Martinez R., Rowsey J. L., Fraser D. G., Eckhardt B. A., Khosla S., Farr J. N., et al. . (2020). LPS-induced premature osteocyte senescence: Implications in inflammatory alveolar bone loss and periodontal disease pathogenesis. Bone 132, 115220. doi: 10.1016/j.bone.2019.115220 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

