Protection against N. gonorrhoeae induced by OMV-based meningococcal vaccines are associated with cross-species directed humoral and cellular immune responses
- PMID: 40292302
- PMCID: PMC12021806
- DOI: 10.3389/fimmu.2025.1539795
Protection against N. gonorrhoeae induced by OMV-based meningococcal vaccines are associated with cross-species directed humoral and cellular immune responses
Abstract
Introduction: Limited protective immunologic responses to natural N. gonorrhoeae infection and a lack of knowledge about mechanisms of protection have hampered development of an effective vaccine. Recent studies in humans and mice have found meningococcal outer membrane vesicle-containing vaccines (OMV) induce cross species immune responses against gonococci and are associated with protection. The exact mechanisms or how humoral and cellular immunity are related to protection, remain unclear.
Methods: To study this, we immunized mice with two meningococcal OMV-containing vaccines known to accelerate clearance of N. gonorrhoeae, 4CMenB and OMV from an engineered N. meningitidis strain lacking major surface antigens PorA, PorB, and Rmp (MC58 ΔABR). We assessed serologic and cellular immune signatures associated with these immunizations and assessed bacterial clearance in the mice using a vaginal/cervical gonococcal infection model.
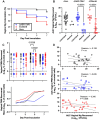
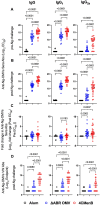
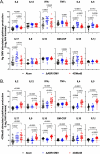
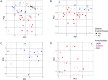
Results: Mice immunized with 4CMenB or MC58 ΔABR demonstrated shortened courses of recovery of vaginal N. gonorrhoeae compared to control mice immunized with alum alone. Vaccination with 4CMenB or MC58ΔABR OMV elicited serum and vaginal cross-reactive anti-Ng-OMV antibody responses that were augmented after vaginal challenge with N. gonorrhoeae. Further, splenocytes in 4CMenB and MC58 ΔABR immunized mice exhibited elevated cytokine production after restimulation with heterologous N. gonorrhoeae OMV when compared to splenocytes from Alum immunized mice. We further tested for correlations between bacterial burden and the measured anti-gonococcal immune responses within each vaccination group and found different immunologic parameters associated with reduced bacterial burden for each vaccine.
Discussion: Our findings suggest the cross-protection against gonococcal infection induced by different meningococcal OMV vaccines is likely multifactorial and mediated by different humoral and cellular immune responses induced by these two vaccines.
Keywords: Neisseria gonorrhoeae; Neisseria meningitidis; correlates of protection; outer membrane vesicle (OMV); vaccine.
Copyright © 2025 Zhu, Waltmann, Little, Connolly, Matthias, Thomas, Gray, Sikora, Criss, Bash, Macintyre, Jerse and Duncan.
Conflict of interest statement
JD has a spouse who is employed by GlaxoSmithKline GSK, the manufacturer of the 4CMenB vaccine, which was utilized in this study. Neither the author’s spouse nor GSK was involved in funding, designing, conducting, analyzing the research reported in this manuscript. JD acknowledges that there is a potential conflict of interest related to the employment status of his spouse with GSK and attests that the research conducted and reported in this manuscript is free of any bias that might be associated with the commercial goals of GSK. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




Update of
-
Protection against N. gonorrhoeae induced by OMV-based Meningococcal Vaccines are associated with cross-species directed humoral and cellular immune responses.bioRxiv [Preprint]. 2024 Nov 30:2024.11.29.626107. doi: 10.1101/2024.11.29.626107. bioRxiv. 2024. Update in: Front Immunol. 2025 Apr 11;16:1539795. doi: 10.3389/fimmu.2025.1539795. PMID: 39651121 Free PMC article. Updated. Preprint.
Similar articles
-
Protection against N. gonorrhoeae induced by OMV-based Meningococcal Vaccines are associated with cross-species directed humoral and cellular immune responses.bioRxiv [Preprint]. 2024 Nov 30:2024.11.29.626107. doi: 10.1101/2024.11.29.626107. bioRxiv. 2024. Update in: Front Immunol. 2025 Apr 11;16:1539795. doi: 10.3389/fimmu.2025.1539795. PMID: 39651121 Free PMC article. Updated. Preprint.
-
Adjuvant-dependent impacts on vaccine-induced humoral responses and protection in preclinical models of nasal and genital colonization by pathogenic Neisseria.Vaccine. 2025 Feb 27;48:126709. doi: 10.1016/j.vaccine.2025.126709. Epub 2025 Jan 15. Vaccine. 2025. PMID: 39817984
-
The serogroup B meningococcal outer membrane vesicle-based vaccine 4CMenB induces cross-species protection against Neisseria gonorrhoeae.PLoS Pathog. 2020 Dec 8;16(12):e1008602. doi: 10.1371/journal.ppat.1008602. eCollection 2020 Dec. PLoS Pathog. 2020. PMID: 33290434 Free PMC article.
-
Predicted vs observed effectiveness of outer membrane vesicle (OMV) vaccines against meningococcal serogroup B disease: Systematic review.J Infect. 2017 Aug;75(2):81-94. doi: 10.1016/j.jinf.2017.05.001. Epub 2017 May 6. J Infect. 2017. PMID: 28487177
-
Vaccine effectiveness and impact of meningococcal vaccines against gonococcal infections: A systematic review and meta-analysis.J Infect. 2024 Sep;89(3):106225. doi: 10.1016/j.jinf.2024.106225. Epub 2024 Jul 8. J Infect. 2024. PMID: 38986746
References
-
- Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, et al. . Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother. (2011) 55:3538–45. doi: 10.1128/aac.00325-11 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

